Absence of cell surface expression of human ACE leads to perinatal death
- PMID: 24163131
- PMCID: PMC3929087
- DOI: 10.1093/hmg/ddt535
Absence of cell surface expression of human ACE leads to perinatal death
Abstract
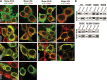
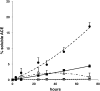
Renal tubular dysgenesis (RTD) is a recessive autosomal disease characterized most often by perinatal death. It is due to the inactivation of any of the major genes of the renin-angiotensin system (RAS), one of which is the angiotensin I-converting enzyme (ACE). ACE is present as a tissue-bound enzyme and circulates in plasma after its solubilization. In this report, we present the effect of different ACE mutations associated with RTD on ACE intracellular trafficking, secretion and enzymatic activity. One truncated mutant, R762X, responsible for neonatal death was found to be an enzymatically active, secreted form, not inserted in the plasma membrane. In contrast, another mutant, R1180P, was compatible with life after transient neonatal renal insufficiency. This mutant was located at the plasma membrane and rapidly secreted. These results highlight the importance of tissue-bound ACE versus circulating ACE and show that the total absence of cell surface expression of ACE is incompatible with life. In addition, two missense mutants (W594R and R828H) and two truncated mutants (Q1136X and G1145AX) were also studied. These mutants were neither inserted in the plasma membrane nor secreted. Finally, the structural implications of these ACE mutations were examined by molecular modelling, which suggested some important structural alterations such as disruption of intra-molecular non-covalent interactions (e.g. salt bridges).
Figures




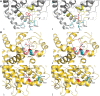
References
-
- Bernstein K.E., Ong F.S., Blackwell W.L., Shah K.H., Giani J.F., Gonzalez-Villalobos R.A., Shen X.Z., Fuchs S., Touyz R.M. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 2013;65:1–46. doi:10.1124/pr.112.006809. - DOI - PMC - PubMed
-
- Wei L., Alhenc-Gelas F., Corvol P., Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991;266:9002–9008. - PubMed
-
- Jaspard E., Wei L., Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J. Biol. Chem. 1993;268:9496–9503. - PubMed
-
- Rousseau A., Michaud A., Chauvet M.T., Lenfant M., Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J. Biol. Chem. 1995;270:3656–3661. doi:10.1074/jbc.270.8.3656. - DOI - PubMed
-
- Ramchandran R., Sen I. Cleavage processing of angiotensin-converting enzyme by a membrane-associated metalloprotease. Biochemistry. 1995;34:12645–12652. doi:10.1021/bi00039a021. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

