Nonsense-mediated decay of alternative precursor mRNA splicing variants is a major determinant of the Arabidopsis steady state transcriptome
- PMID: 24163313
- PMCID: PMC3877825
- DOI: 10.1105/tpc.113.115485
Nonsense-mediated decay of alternative precursor mRNA splicing variants is a major determinant of the Arabidopsis steady state transcriptome
Abstract
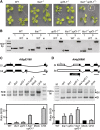
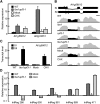
The nonsense-mediated decay (NMD) surveillance pathway can recognize erroneous transcripts and physiological mRNAs, such as precursor mRNA alternative splicing (AS) variants. Currently, information on the global extent of coupled AS and NMD remains scarce and even absent for any plant species. To address this, we conducted transcriptome-wide splicing studies using Arabidopsis thaliana mutants in the NMD factor homologs UP FRAMESHIFT1 (UPF1) and UPF3 as well as wild-type samples treated with the translation inhibitor cycloheximide. Our analyses revealed that at least 17.4% of all multi-exon, protein-coding genes produce splicing variants that are targeted by NMD. Moreover, we provide evidence that UPF1 and UPF3 act in a translation-independent mRNA decay pathway. Importantly, 92.3% of the NMD-responsive mRNAs exhibit classical NMD-eliciting features, supporting their authenticity as direct targets. Genes generating NMD-sensitive AS variants function in diverse biological processes, including signaling and protein modification, for which NaCl stress-modulated AS-NMD was found. Besides mRNAs, numerous noncoding RNAs and transcripts derived from intergenic regions were shown to be NMD responsive. In summary, we provide evidence for a major function of AS-coupled NMD in shaping the Arabidopsis transcriptome, having fundamental implications in gene regulation and quality control of transcript processing.
Figures





References
-
- Arciga-Reyes L., Wootton L., Kieffer M., Davies B. (2006). UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J. 47: 480–489 - PubMed
-
- Azzalin C.M., Lingner J. (2006). The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 16: 433–439 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

