Polarized Enhanced Backscattering Spectroscopy for Characterization of Biological Tissues at Subdiffusion Length-scales
- PMID: 24163574
- PMCID: PMC3806115
- DOI: 10.1109/JSTQE.2011.2173659
Polarized Enhanced Backscattering Spectroscopy for Characterization of Biological Tissues at Subdiffusion Length-scales
Abstract
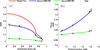
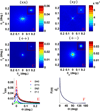
Since the early 1980's, the enhanced backscattering (EBS) phenomenon has been well-studied in a large variety of non-biological materials. Yet, until recently the use of conventional EBS for the characterization of biological tissue has been fairly limited. In this work we detail the unique ability of EBS to provide spectroscopic, polarimetric, and depth-resolved characterization of biological tissue using a simple backscattering instrument. We first explain the experimental and numerical procedures used to accurately measure and model the full azimuthal EBS peak shape in biological tissue. Next we explore the peak shape and height dependencies for different polarization channels and spatial coherence of illumination. We then illustrate the extraordinary sensitivity of EBS to the shape of the scattering phase function using suspensions of latex microspheres. Finally, we apply EBS to biological tissue samples in order to measure optical properties and observe the spatial length-scales at which backscattering is altered in early colon carcinogenesis.
Keywords: Enhanced backscattering; backscattering spectroscopy; cancer detection; polarized light Monte Carlo.
Figures
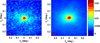



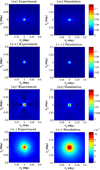
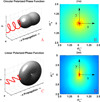


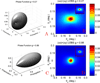

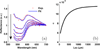

References
-
- Kuga Y, Ishimaru A. Retroreflectance from a Dense Distribution of Spherical-Particles. J Opt Soc Am A. 1984;vol. 1:831–835.
-
- Tsang L, Ishimaru A. Backscattering Enhancement of Random Discrete Scatterers. J Opt Soc Am A. 1984;vol. 1:836–839.
-
- Wolf PE, Maret G. Weak Localization and Coherent Backscattering of Photons in Disordered Media. Phys Rev Lett. 1985;vol. 55:2696–2699. - PubMed
-
- Van albada MP, Lagendijk A. Observation of Weak Localization of Light in a Random Medium. Phys Rev Lett. 1985;vol. 55:2692–2695. - PubMed
-
- Akkermans E, Wolf PE, Maynard R. Coherent Backscattering of Light by Disordered Media - Analysis of the Peak Line-Shape. Phys Rev Lett. 1986 Apr 7;vol. 56:1471–1474. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
