Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications
- PMID: 24163634
- PMCID: PMC3791836
- DOI: 10.1155/2013/863157
Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications
Abstract
Autogenous bone grafting remains a gold standard for the reconstruction critical-sized bone defects in the craniomaxillofacial region. Nevertheless, this graft procedure has several disadvantages such as restricted availability, donor-site morbidity, and limitations in regard to fully restoring the complicated three-dimensional structures in the craniomaxillofacial bone. The ultimate goal of craniomaxillofacial bone reconstruction is the regeneration of the physiological bone that simultaneously fulfills both morphological and functional restorations. Developments of tissue engineering in the last two decades have brought such a goal closer to reality. In bone tissue engineering, the scaffolds are fundamental, elemental and mesenchymal stem cells/osteoprogenitor cells and bioactive factors. A variety of scaffolds have been developed and used as spacemakers, biodegradable bone substitutes for transplanting to the new bone, matrices of drug delivery system, or supporting structures enhancing adhesion, proliferation, and matrix production of seeded cells according to the circumstances of the bone defects. However, scaffolds to be clinically completely satisfied have not been developed yet. Development of more functional scaffolds is required to be applied widely to cranio-maxillofacial bone defects. This paper reviews recent trends of scaffolds for crania-maxillofacial bone tissue engineering, including our studies.
Figures

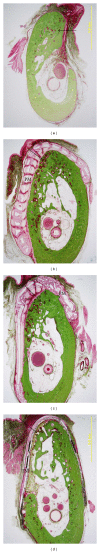
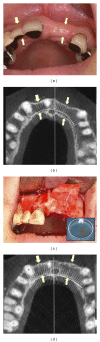

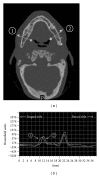
References
-
- Jewer DD, Boyd JB, Manktelow RT, et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plastic and Reconstructive Surgery. 1989;84(3):391–403. - PubMed
-
- Wei F-C, Seah C-S, Tsai Y-C, Liu S-J, Tsai M-S, Hidalgo DA. Fibula osteoseptocutaneous flap for reconstruction of composite mandibular defects. Plastic and Reconstructive Surgery. 1994;93(2):294–306. - PubMed
-
- Schöning H, Emshoff R. Primary temporary AO plate reconstruction of the mandible. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 1998;86(6):667–672. - PubMed
-
- Urken ML, Bridger AG, Zur KB, Genden EM. The scapular osteofasciocutaneous flap: a 12-year experience. Archives of Otolaryngology. 2001;127(7):862–869. - PubMed
-
- Goh BT, Lee S, Tideman H, Stoelinga PJW. Mandibular reconstruction in adults: a review. International Journal of Oral and Maxillofacial Surgery. 2008;37(7):597–605. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

