Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer
- PMID: 24163750
- PMCID: PMC3804878
- DOI: 10.3978/j.issn.2072-1439.2013.08.52
Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer
Abstract
Advances in the treatment of non-small cell lung cancer (NSCLC) over the last decade have predominantly involved the development of therapies directed at molecular targets such as mutations in the epidermal growth factor receptor (EGFR) or rearrangements in the anaplastic lymphoma kinase (ALK) gene. Other targets have been discovered at low frequency, with multiple agents approved or in development for treatment of these rare molecular subtypes. The tumour microenvironment has also provided opportunities for therapies targeting angiogenesis and the host immune response. This review will provide an overview of current targeted therapies in NSCLC and promising treatment approaches on the horizon.
Keywords: Non-small-cell lung carcinoma (NSCLC); anaplastic lymphoma kinase (ALK); epidermal growth factor receptor (EGFR); immunotherapy; molecular targeted therapy.
Figures
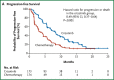

References
-
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol 2003;21:2237-46 - PubMed
-
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58 - PubMed
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39 - PubMed
-
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
