Phytoestrogens in postmenopause: the state of the art from a chemical, pharmacological and regulatory perspective
- PMID: 24164197
- PMCID: PMC3963458
- DOI: 10.2174/09298673113206660297
Phytoestrogens in postmenopause: the state of the art from a chemical, pharmacological and regulatory perspective
Abstract
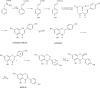
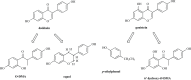
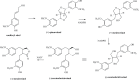
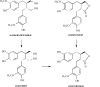
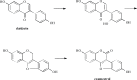
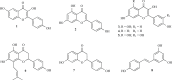
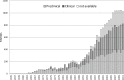
Phytoestrogens represent a diverse group of non-steroidal natural products, which seem to have some oestrogenic effects and are often marketed as food supplements. Population exposed to phytoestrogens is potentially increasing, in part because an unfavourable risk-benefit profile of Hormone Replacement Therapy (HRT) for prolonged treatments (e.g., osteoporosis prevention) highlighted by the publication of the Women Health Initiative (WHI) trial in 2002, but also because many post-menopausal women often perceived phytoestrogens in food supplements as a safer alternative than HRT. Despite of increasing preclinical and clinical studies in the past decade, appealing evidence is still lacking to support the overall positive risk-benefit profile of phytoestrogens. Their status as food supplements seems to discourage studies to obtain new evidence, and the chance to buy them by user's initiative make it difficult to survey their prevalence and pattern of use. The aim of the present review is to: (a) outline the clinical scenario underlying the increased interest on phytoestrogens, by overviewing the evolution of the evidence on HRT and its main therapeutic goals (e.g., menopausal symptoms relief, chemoprevention, osteoporosis prevention); (b) address the chemical and pharmacological features (e.g. chemical structure, botanical sources, mechanism of action) of the main compounds (e.g., isoflavones, lignans, coumestans); (c) describe the clinical evidence on potential therapeutic applications; (d) put available evidence on their riskbenefit profile in a regulatory perspective, in light of the recent regulation on health claims of food supplements.
Figures

References
-
- Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women.The Framingham Study. N. Engl. J Med. 1987;317(19):1169–1174. - PubMed
-
- Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women.Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 1995;122(1):9–16. - PubMed
-
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann. Intern. Med. 2000;133(12):933–941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

