Actin dynamics in growth cone motility and navigation
- PMID: 24164353
- PMCID: PMC3980044
- DOI: 10.1111/jnc.12506
Actin dynamics in growth cone motility and navigation
Abstract
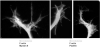
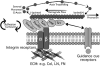
Motile growth cones lead growing axons through developing tissues to synaptic targets. These behaviors depend on the organization and dynamics of actin filaments that fill the growth cone leading margin [peripheral (P-) domain]. Actin filament organization in growth cones is regulated by actin-binding proteins that control all aspects of filament assembly, turnover, interactions with other filaments and cytoplasmic components, and participation in producing mechanical forces. Actin filament polymerization drives protrusion of sensory filopodia and lamellipodia, and actin filament connections to the plasma membrane link the filament network to adhesive contacts of filopodia and lamellipodia with other surfaces. These contacts stabilize protrusions and transduce mechanical forces generated by actomyosin activity into traction that pulls an elongating axon along the path toward its target. Adhesive ligands and extrinsic guidance cues bind growth cone receptors and trigger signaling activities involving Rho GTPases, kinases, phosphatases, cyclic nucleotides, and [Ca++] fluxes. These signals regulate actin-binding proteins to locally modulate actin polymerization, interactions, and force transduction to steer the growth cone leading margin toward the sources of attractive cues and away from repellent guidance cues.
Keywords: actin; actin-binding proteins; axon guidance; growth cone.
© 2013 International Society for Neurochemistry.
Figures




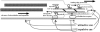


References
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

