A screen of zebrafish mutants identifies ethanol-sensitive genetic loci
- PMID: 24164477
- PMCID: PMC3959233
- DOI: 10.1111/acer.12286
A screen of zebrafish mutants identifies ethanol-sensitive genetic loci
Abstract
Background: Fetal alcohol spectrum disorders (FASD) are a highly variable set of phenotypes caused by fetal alcohol exposure. Numerous factors influence FASD phenotypes, including genetics. The zebrafish is a powerful vertebrate model system with which to identify these genetic factors. Many zebrafish mutants are housed at the Zebrafish International Resource Center (ZIRC). These mutants are readily accessible and an excellent source to screen for ethanol (EtOH)-sensitive developmental structural mutants.
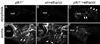
Methods: We screened mutants obtained from ZIRC for sensitivity to EtOH teratogenesis. Embryos were treated with 1% EtOH (41 mM tissue levels) from 6 hours postfertilization onward. Levels of apoptosis were evaluated at 24 hours postfertilization. At 4 days postfertilization, the craniofacial skeleton, peripheral axon projections, and sensory neurons of neuromasts were examined. Fish were genotyped to determine whether there were phenotype/genotype correlations.
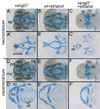
Results: Five of 20 loci interacted with EtOH. Notable among these was that vangl2, involved in convergent extension movements of the embryonic axis, interacted strongly with EtOH. Untreated vangl2 mutants had normal craniofacial morphology, while severe midfacial defects including synophthalmia and narrowing of the palatal skeleton were found in all EtOH-treated mutants and a low percentage of heterozygotes. The cell cycle gene, plk1, also interacted strongly with EtOH. Untreated mutants have slightly elevated levels of apoptosis and loss of ventral craniofacial elements. Exposure to EtOH results in extensive apoptosis along with loss of neural tissue and the entire craniofacial skeleton. Phenotypes of hinfp, mars, and foxi1 mutants were also exacerbated by EtOH.
Conclusions: Our results provide insight into the gene-EtOH interactions that may underlie EtOH teratogenesis. They support previous findings that EtOH disrupts elongation of the embryonic axis. Importantly, these results show that the zebrafish is an efficient model with which to test for gene-EtOH interactions. Understanding these interactions will be crucial to understanding of the FASD variation.
Keywords: Craniofacial; FASD; Genetic Screen; Zebrafish.
Copyright © 2013 by the Research Society on Alcoholism.
Figures






Similar articles
-
Zebrafish models of fetal alcohol spectrum disorders.Genesis. 2021 Nov;59(11):e23460. doi: 10.1002/dvg.23460. Epub 2021 Nov 5. Genesis. 2021. PMID: 34739740 Free PMC article. Review.
-
Novel Ethanol-Sensitive Mutants Identified in an F3 Forward Genetic Screen.Alcohol Clin Exp Res. 2020 Jan;44(1):56-65. doi: 10.1111/acer.14240. Epub 2019 Dec 17. Alcohol Clin Exp Res. 2020. PMID: 31742718 Free PMC article.
-
Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD.Development. 2013 Aug;140(15):3254-65. doi: 10.1242/dev.094938. Development. 2013. PMID: 23861062 Free PMC article.
-
Exposure to ethanol leads to midfacial hypoplasia in a zebrafish model of FASD via indirect interactions with the Shh pathway.BMC Biol. 2021 Jul 1;19(1):134. doi: 10.1186/s12915-021-01062-9. BMC Biol. 2021. PMID: 34210294 Free PMC article.
-
Diving into the world of alcohol teratogenesis: a review of zebrafish models of fetal alcohol spectrum disorder.Biochem Cell Biol. 2018 Apr;96(2):88-97. doi: 10.1139/bcb-2017-0122. Epub 2017 Aug 17. Biochem Cell Biol. 2018. PMID: 28817785 Free PMC article. Review.
Cited by
-
Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies.Alcohol Res. 2015;37(1):97-108. Alcohol Res. 2015. PMID: 26259091 Free PMC article. Review.
-
Zebrafish models of fetal alcohol spectrum disorders.Genesis. 2021 Nov;59(11):e23460. doi: 10.1002/dvg.23460. Epub 2021 Nov 5. Genesis. 2021. PMID: 34739740 Free PMC article. Review.
-
Transient exposure to ethanol during zebrafish embryogenesis results in defects in neuronal differentiation: an alternative model system to study FASD.PLoS One. 2014 Nov 10;9(11):e112851. doi: 10.1371/journal.pone.0112851. eCollection 2014. PLoS One. 2014. PMID: 25383948 Free PMC article.
-
Developmental age strengthens barriers to ethanol accumulation in zebrafish.Alcohol. 2014 Sep;48(6):595-602. doi: 10.1016/j.alcohol.2014.06.003. Epub 2014 Jun 8. Alcohol. 2014. PMID: 25012627 Free PMC article.
-
Comparison of molecular marker expression in early zebrafish brain development following chronic ethanol or morpholino treatment.Exp Brain Res. 2017 Aug;235(8):2413-2423. doi: 10.1007/s00221-017-4977-5. Epub 2017 May 10. Exp Brain Res. 2017. PMID: 28493069 Free PMC article.
References
-
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol. 2004;26(6):737–743. - PubMed
-
- Blader P, Strahle U. Ethanol impairs migration of the prechordal plate in the zebrafish embryo. Dev Biol. 1998;201(2):185–201. - PubMed
-
- Cartwright MM, Smith SM. Stage-dependent effects of ethanol on cranial neural crest cell development: partial basis for the phenotypic variations observed in fetal alcohol syndrome. Alcohol Clin Exp Res. 1995;19(6):1454–1462. - PubMed
-
- Cavieres MF, Smith SM. Genetic and Developmental Modulation of Cardiac Deficits in Prenatal Alcohol Exposure. Alcoholism: Clinical and Experimental Research. 2000;24(1):102–109. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

