Transcriptional downregulation of agr expression in Staphylococcus aureus during growth in human serum can be overcome by constitutively active mutant forms of the sensor kinase AgrC
- PMID: 24164684
- PMCID: PMC4274972
- DOI: 10.1111/1574-6968.12309
Transcriptional downregulation of agr expression in Staphylococcus aureus during growth in human serum can be overcome by constitutively active mutant forms of the sensor kinase AgrC
Abstract
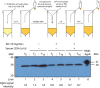
The temporal and cell density-dependent regulation of expression of virtually all the Staphylococcus aureus virulon is under the control of the agr (accessory gene regulatory) operon. The expression of the agr operon is subject to transcriptional regulation by the AgrA/C two-component response regulator/sensor kinase pair. During bacteraemia, a frequent syndrome caused by methicillin-resistant S. aureus (MRSA), the transcriptional downregulation of agr expression has been attributed to the sequestration of the quorum-signalling molecule auto-inducing peptide (AIP) by the human serum component apolipoprotein B as part of an innate immune response to infection. However, it is not known whether transcriptional downregulation of agr expression during growth in human serum is additionally subjected to regulation by transcription regulatory proteins that either directly or indirectly affect transcription from the agr operon promoters. Here, using chromosomal fluorescence reporters of agr expression in S. aureus, we show that the transcriptional downregulation of agr expression in human serum can be overcome using constitutive active mutant forms of AgrC. Therefore, it seems that the sequestration of the AIP is likely to be the only mechanism by which the host innate immune response limits agr expression at the transcriptional level to maintain the host-pathogen balance towards a noninvasive outcome.
Keywords: GFP transcriptional reporters; transcription regulation; two-component systems.
© 2013 The Authors. FEMS Microbiology Letters Published by John Wiley & Sons Ltd on behalf of the Federation of European Microbiological Societies.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

