Crumbs3 is essential for proper epithelial development and viability
- PMID: 24164893
- PMCID: PMC3911272
- DOI: 10.1128/MCB.00999-13
Crumbs3 is essential for proper epithelial development and viability
Abstract
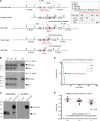
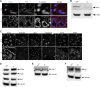
First identified in Drosophila, the Crumbs (Crb) proteins are important in epithelial polarity, apical membrane formation, and tight junction (TJ) assembly. The conserved Crb intracellular region includes a FERM (band 4.1/ezrin/radixin/moesin) binding domain (FBD) whose mammalian binding partners are not well understood and a PDZ binding motif that interacts with mammalian Pals1 (protein associated with lin seven) (also known as MPP5). Pals1 binds Patj (Pals1-associated tight-junction protein), a multi-PDZ-domain protein that associates with many tight junction proteins. The Crb complex also binds the conserved Par3/Par6/atypical protein kinase C (aPKC) polarity cassette that restricts migration of basolateral proteins through phosphorylation. Here, we describe a Crb3 knockout mouse that demonstrates extensive defects in epithelial morphogenesis. The mice die shortly after birth, with cystic kidneys and proteinaceous debris throughout the lungs. The intestines display villus fusion, apical membrane blebs, and disrupted microvilli. These intestinal defects phenocopy those of Ezrin knockout mice, and we demonstrate an interaction between Crumbs3 and ezrin. Taken together, our data indicate that Crumbs3 is crucial for epithelial morphogenesis and plays a role in linking the apical membrane to the underlying ezrin-containing cytoskeleton.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK089933/DK/NIDDK NIH HHS/United States
- DK069605/DK/NIDDK NIH HHS/United States
- DK073722/DK/NIDDK NIH HHS/United States
- R01 DK065850/DK/NIDDK NIH HHS/United States
- R01 DK073722/DK/NIDDK NIH HHS/United States
- DK06914/DK/NIDDK NIH HHS/United States
- DK089119/DK/NIDDK NIH HHS/United States
- R56 DK089933/DK/NIDDK NIH HHS/United States
- HL079339/HL/NHLBI NIH HHS/United States
- DK089933/DK/NIDDK NIH HHS/United States
- R01 HL079339/HL/NHLBI NIH HHS/United States
- DK065850/DK/NIDDK NIH HHS/United States
- R01 DK069605/DK/NIDDK NIH HHS/United States
- K08 DK089119/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
