Are vancomycin trough concentrations adequate for optimal dosing?
- PMID: 24165176
- PMCID: PMC3910745
- DOI: 10.1128/AAC.01653-13
Are vancomycin trough concentrations adequate for optimal dosing?
Abstract
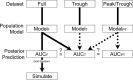
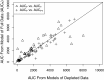
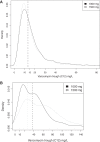
The current vancomycin therapeutic guidelines recommend the use of only trough concentrations to manage the dosing of adults with Staphylococcus aureus infections. Both vancomycin efficacy and toxicity are likely to be related to the area under the plasma concentration-time curve (AUC). We assembled richly sampled vancomycin pharmacokinetic data from three studies comprising 47 adults with various levels of renal function. With Pmetrics, the nonparametric population modeling package for R, we compared AUCs estimated from models derived from trough-only and peak-trough depleted versions of the full data set and characterized the relationship between the vancomycin trough concentration and AUC. The trough-only and peak-trough depleted data sets underestimated the true AUCs compared to the full model by a mean (95% confidence interval) of 23% (11 to 33%; P = 0.0001) and 14% (7 to 19%; P < 0.0001), respectively. In contrast, using the full model as a Bayesian prior with trough-only data allowed 97% (93 to 102%; P = 0.23) accurate AUC estimation. On the basis of 5,000 profiles simulated from the full model, among adults with normal renal function and a therapeutic AUC of ≥400 mg · h/liter for an organism for which the vancomycin MIC is 1 mg/liter, approximately 60% are expected to have a trough concentration below the suggested minimum target of 15 mg/liter for serious infections, which could result in needlessly increased doses and a risk of toxicity. Our data indicate that adjustment of vancomycin doses on the basis of trough concentrations without a Bayesian tool results in poor achievement of maximally safe and effective drug exposures in plasma and that many adults can have an adequate vancomycin AUC with a trough concentration of <15 mg/liter.
Figures
References
-
- Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82–98. 10.2146/ajhp080434 - DOI - PubMed
-
- Suzuki Y, Kawasaki K, Sato Y, Tokimatsu I, Itoh H, Hiramatsu K, Takeyama M, Kadota J-I. 2012. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy 58:308–312. 10.1159/000343162 - DOI - PubMed
-
- Fitzsimmons WE, Postelnick MJ, Tortorice PV. 1988. Survey of vancomycin monitoring guidelines in Illinois hospitals. Drug Intell. Clin. Pharm. 22:598–600 - PubMed
-
- Gawronski KM, Goff DA, Brown J, Khadem TM, Bauer KA. 2013. A stewardship program's retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin. Ther. 35:772–779. 10.1016/j.clinthera.2013.05.008 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

