Activation of amygdala opioid receptors by electroacupuncture of Feng-Chi (GB20) acupoints exacerbates focal epilepsy
- PMID: 24165229
- PMCID: PMC3816151
- DOI: 10.1186/1472-6882-13-290
Activation of amygdala opioid receptors by electroacupuncture of Feng-Chi (GB20) acupoints exacerbates focal epilepsy
Abstract
Background: The effect of seizure suppression by acupuncture of Feng-Chi (GB20) acupoints has been documented in the ancient Chinese literature, Lingshu Jing (Classic of the Miraculous Pivot), however, there is a lack of scientific evidence to prove it. This current study was designed to elucidate the effect of electroacupuncture (EA) stimulation of bilateral Feng-Chi (GB20) acupoints on the epileptic activity by employing an animal model of focal epilepsy.
Methods: Administration of pilocarpine into the left central nucleus of amygdala (CeA) induced the focal epilepsy in rats. Rats received a 30-min 100 Hz EA stimulation of bilateral Feng-Chi acupoints per day, beginning at 30 minutes before the dark period and performing in three consecutive days. The broad-spectrum opioid receptor antagonist (naloxone), μ-receptor antagonist (naloxonazine), δ-receptor antagonist (naltrindole) and κ-receptor antagonist (nor-binaltorphimine) were administered directly into the CeA to elucidate the involvement of CeA opioid receptors in the EA effect.
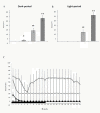
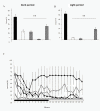
Results: High-frequency (100 Hz) EA stimulation of bilateral Feng-Chi acupoints did not suppress the pilocarpine-induced epileptiform electroencephalograms (EEGs), whereas it further increased the duration of epileptiform EEGs. We also observed that epilepsy occurred while 100 Hz EA stimulation of Feng-Chi acupoints was delivered into naïve rats. EA-induced augmentation of epileptic activity was blocked by microinjection of naloxone, μ- (naloxonazine), κ- (nor-binaltorphimine) or δ-receptor antagonists (natrindole) into the CeA, suggesting that activation of opioid receptors in the CeA mediates EA-exacerbated epilepsy.
Conclusions: The present study suggests that high-frequency (100 Hz) EA stimulation of bilateral Feng-Chi acupoints has no effect to protect against pilocarpine-induced focal epilepsy; in contrast, EA further exacerbated focal epilepsy induced by pilocarpine. Opioid receptors in the CeA mediated EA-induced exacerbation of focal epilepsy.
Figures



Similar articles
-
Low-frequency electroacupuncture suppresses focal epilepsy and improves epilepsy-induced sleep disruptions.J Biomed Sci. 2015 Jul 7;22(1):49. doi: 10.1186/s12929-015-0145-z. J Biomed Sci. 2015. PMID: 26150021 Free PMC article.
-
Amygdala opioid receptors mediate the electroacupuncture-induced deterioration of sleep disruptions in epilepsy rats.J Biomed Sci. 2013 Nov 12;20(1):85. doi: 10.1186/1423-0127-20-85. J Biomed Sci. 2013. PMID: 24215575 Free PMC article.
-
[Parametric optimization of electroacupuncture against bone-cancer pain in rats and its intervention on mRNA expression of opioid receptor and precursor].Zhongguo Zhen Jiu. 2015 Feb;35(2):161-8. Zhongguo Zhen Jiu. 2015. PMID: 25854026 Chinese.
-
Acupoint-specific, frequency-dependent, and improved insulin sensitivity hypoglycemic effect of electroacupuncture applied to drug-combined therapy studied by a randomized control clinical trial.Evid Based Complement Alternat Med. 2014;2014:371475. doi: 10.1155/2014/371475. Epub 2014 Jun 16. Evid Based Complement Alternat Med. 2014. PMID: 25024728 Free PMC article. Review.
-
Electroacupuncture stimulation to modulate neural oscillations in promoting neurological rehabilitation.Brain Res. 2024 Jan 1;1822:148642. doi: 10.1016/j.brainres.2023.148642. Epub 2023 Oct 24. Brain Res. 2024. PMID: 37884179 Review.
Cited by
-
Manipulation of Epileptiform Electrocorticograms (ECoGs) and Sleep in Rats and Mice by Acupuncture.J Vis Exp. 2016 Dec 22;(118):54896. doi: 10.3791/54896. J Vis Exp. 2016. PMID: 28060294 Free PMC article.
-
Low-frequency electroacupuncture suppresses focal epilepsy and improves epilepsy-induced sleep disruptions.J Biomed Sci. 2015 Jul 7;22(1):49. doi: 10.1186/s12929-015-0145-z. J Biomed Sci. 2015. PMID: 26150021 Free PMC article.
References
-
- DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, Reed R, Collin S, Tecoma E, Morris GL, Vaughn B, Naritoku DK, Henry T, Labar D, Gilmartin R, Labiner D, Osorio I, Ristanovic R, Jones J, Murphy J, Ney G, Wheless J, Lewis P, Heck C. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. - DOI - PubMed
-
- Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1990;31:S7–S19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

