Identification of properties important to protein aggregation using feature selection
- PMID: 24165390
- PMCID: PMC3819749
- DOI: 10.1186/1471-2105-14-314
Identification of properties important to protein aggregation using feature selection
Abstract
Background: Protein aggregation is a significant problem in the biopharmaceutical industry (protein drug stability) and is associated medically with over 40 human diseases. Although a number of computational models have been developed for predicting aggregation propensity and identifying aggregation-prone regions in proteins, little systematic research has been done to determine physicochemical properties relevant to aggregation and their relative importance to this important process. Such studies may result in not only accurately predicting peptide aggregation propensities and identifying aggregation prone regions in proteins, but also aid in discovering additional underlying mechanisms governing this process.
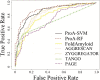
Results: We use two feature selection algorithms to identify 16 features, out of a total of 560 physicochemical properties, presumably important to protein aggregation. Two predictors (ProA-SVM and ProA-RF) using selected features are built for predicting peptide aggregation propensity and identifying aggregation prone regions in proteins. Both methods are compared favourably to other state-of-the-art algorithms in cross validation. The identified important properties are fairly consistent with previous studies and bring some new insights into protein and peptide aggregation. One interesting new finding is that aggregation prone peptide sequences have similar properties to signal peptide and signal anchor sequences.
Conclusions: Both predictors are implemented in a freely available web application (http://www.abl.ku.edu/ProA/). We suggest that the quaternary structure of protein aggregates, especially soluble oligomers, may allow the formation of new molecular recognition signals that guide aggregate targeting to specific cellular sites.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

