Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease
- PMID: 24165906
- PMCID: PMC4263162
- DOI: 10.1002/jcph.215
Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease
Abstract
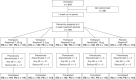
The aim of the study was to characterize pharmacokinetics of tiotropium solution 5 µg compared to powder 18 µg and assess dose-dependency of tiotropium solution pharmacodynamics in comparison to placebo. In total 154 patients with chronic obstructive pulmonary disease (COPD) were included in this multicenter, randomized, double-blind within-solution (1.25, 2.5, 5 µg, and placebo), and open-label powder 18 µg, crossover study, including 4-week treatment periods. Primary end points were peak plasma concentration (Cmax,ss ), and area under the plasma concentration-time profile (AUC0-6h,ss ), both at steady state. The pharmacodynamic response was assessed by serial spirometry (forced expiratory volume in 1 second/forced vital capacity). Safety was evaluated as adverse events and by electrocardiogram/Holter. Tiotropium was rapidly absorbed with a median tmax,ss of 5-7 minutes postdosing for both devices. The gMean ratio of solution 5 µg over powder 18 µg was 81% (90% confidence interval, 73-89%) for Cmax,ss and 76% (70-82%) for AUC0-6h,ss , indicating that bioequivalence was not established. Dose ordering for bronchodilation was observed. Powder 18 µg and solution 5 µg were most effective, providing comparable bronchodilation. All treatments were well tolerated with no apparent relation to dose or device. Comparable bronchodilator efficacy to powder18 µg at lower systemic exposure supports tiotropium solution 5 µg for maintenance treatment of COPD.
Trial registration: ClinicalTrials.gov NCT01222533.
Keywords: COPD; pharmacodynamics; pharmacokinetics; tiotropium HandiHaler®; tiotropium Respimat® SMI.
© 2013 The Authors. The Journal of Clinical Pharmacology Published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures


References
-
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. - PubMed
-
- From the Global Strategy for the Diagnosis Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available from: http://www.goldcopd.org/. Last accessed April 2011. - PubMed
-
- Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460–1472. - PubMed
-
- Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128(3):1168–1178. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

