New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis
- PMID: 24166207
- PMCID: PMC6701984
- DOI: 10.1002/jat.2949
New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis
Abstract
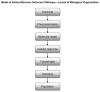
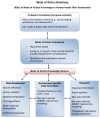
The World Health Organization/International Programme on Chemical Safety mode of action/human relevance framework has been updated to reflect the experience acquired in its application and extend its utility to emerging areas in toxicity testing and non-testing methods. The underlying principles have not changed, but the framework's scope has been extended to enable integration of information at different levels of biological organization and reflect evolving experience in a much broader range of potential applications. Mode of action/species concordance analysis can also inform hypothesis-based data generation and research priorities in support of risk assessment. The modified framework is incorporated within a roadmap, with feedback loops encouraging continuous refinement of fit-for-purpose testing strategies and risk assessment. Important in this construct is consideration of dose-response relationships and species concordance analysis in weight of evidence. The modified Bradford Hill considerations have been updated and additionally articulated to reflect increasing experience in application for cases where the toxicological outcome of chemical exposure is known. The modified framework can be used as originally intended, where the toxicological effects of chemical exposure are known, or in hypothesizing effects resulting from chemical exposure, using information on putative key events in established modes of action from appropriate in vitro or in silico systems and other lines of evidence. This modified mode of action framework and accompanying roadmap and case examples are expected to contribute to improving transparency in explicitly addressing weight of evidence considerations in mode of action/species concordance analysis based on both conventional data sources and evolving methods.
Keywords: adverse outcome pathway; cellular response; human relevance framework; key events; mode of action; modified Bradford Hill considerations; molecular target; species concordance analysis; tissue response; weight of evidence approach.
Copyright © 2013 John Wiley & Sons, Ltd. The World Health Organization retains copyright and all other rights in the manuscript of this article as submitted for publication.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence.J Appl Toxicol. 2014 Jun;34(6):595-606. doi: 10.1002/jat.2984. Epub 2014 Feb 10. J Appl Toxicol. 2014. PMID: 24777878 Free PMC article. Review.
-
IPCS framework for analyzing the relevance of a noncancer mode of action for humans.Crit Rev Toxicol. 2008;38(2):87-96. doi: 10.1080/10408440701749421. Crit Rev Toxicol. 2008. PMID: 18259981 Review.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Procedural application of mode-of-action and human relevance analysis: styrene-induced lung tumors in mice.Crit Rev Toxicol. 2024 Feb;54(2):134-151. doi: 10.1080/10408444.2024.2310600. Epub 2024 Mar 5. Crit Rev Toxicol. 2024. PMID: 38440945 Review.
-
Increasing Scientific Confidence in Adverse Outcome Pathways: Application of Tailored Bradford-Hill Considerations for Evaluating Weight of Evidence.Regul Toxicol Pharmacol. 2015 Aug;72(3):514-37. doi: 10.1016/j.yrtph.2015.04.004. Epub 2015 Apr 8. Regul Toxicol Pharmacol. 2015. PMID: 25863193
Cited by
-
Konzept für die Bewertung von krebserzeugenden Stoffen im bevölkerungsbezogenen Human-Biomonitoring – Stellungnahme der Kommission Human-Biomonitoring des Umweltbundesamtes.Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022 Sep;65(9):951-957. doi: 10.1007/s00103-022-03570-7. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022. PMID: 36048212 German. No abstract available.
-
Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens.Arch Toxicol. 2020 Aug;94(8):2899-2923. doi: 10.1007/s00204-020-02784-5. Epub 2020 Jun 27. Arch Toxicol. 2020. PMID: 32594184 Free PMC article.
-
Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health.EFSA J. 2021 Aug 3;19(8):e06768. doi: 10.2903/j.efsa.2021.6768. eCollection 2021 Aug. EFSA J. 2021. PMID: 34377190 Free PMC article.
-
Specific effects on the thyroid relevant for performing a dietary cumulative risk assessment of pesticide residues: 2024 update.EFSA J. 2024 Mar 18;22(3):e8672. doi: 10.2903/j.efsa.2024.8672. eCollection 2024 Mar. EFSA J. 2024. PMID: 38500786 Free PMC article.
-
Systems Toxicology: Real World Applications and Opportunities.Chem Res Toxicol. 2017 Apr 17;30(4):870-882. doi: 10.1021/acs.chemrestox.7b00003. Epub 2017 Mar 31. Chem Res Toxicol. 2017. PMID: 28362102 Free PMC article.
References
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. - PubMed
-
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 2006;36:781–792. - PubMed
-
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 2008;38:87–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

