The skin is an important bulwark of acquired immunity against intestinal helminths
- PMID: 24166714
- PMCID: PMC3832932
- DOI: 10.1084/jem.20130761
The skin is an important bulwark of acquired immunity against intestinal helminths
Abstract
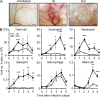
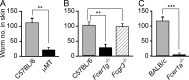
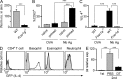
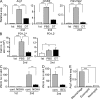
Once animals have experienced a helminthic infection, they often show stronger protective immunity against subsequent infections. Although helminthic infections are well known to elicit Th2-type immune responses, it remains ill-defined where and how acquired protection is executed. Here we show that skin-invading larvae of the intestinal helminth Nippostrongylus brasiliensis are surrounded by skin-infiltrating cells and are prevented from migrating out of infected skin during the second but not the first infection. B cell- or IgE receptor FcεRI-deficient mice showed impaired larval trapping in the skin. Selective ablation of basophils, but not mast cells, abolished the larval trapping, leading to increased worm burden in the lung and hence severe lung injury. Skin-infiltrating basophils produced IL-4 that in turn promoted the generation of M2-type macrophages, leading to the larval trapping in the skin through arginase-1 production. Basophils had no apparent contribution to worm expulsion from the intestine. This study thus reveals a novel mode of acquired antihelminth immunity, in which IgE-armed basophils mediate skin trapping of larvae, thereby limiting lung injury caused by larval migration.
Figures




References
-
- Africa C.M. 1931. Studies on the host relations of Nippostrongylus muris with special reference to age resistance and acquired immunity. J. Parasitol. 18:1–13 10.2307/3271737 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

