Keratins as the main component for the mechanical integrity of keratinocytes
- PMID: 24167246
- PMCID: PMC3831947
- DOI: 10.1073/pnas.1313491110
Keratins as the main component for the mechanical integrity of keratinocytes
Abstract
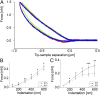
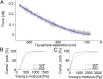
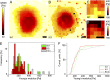
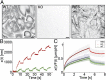
Keratins are major components of the epithelial cytoskeleton and are believed to play a vital role for mechanical integrity at the cellular and tissue level. Keratinocytes as the main cell type of the epidermis express a differentiation-specific set of type I and type II keratins forming a stable network and are major contributors of keratinocyte mechanical properties. However, owing to compensatory keratin expression, the overall contribution of keratins to cell mechanics was difficult to examine in vivo on deletion of single keratin genes. To overcome this problem, we used keratinocytes lacking all keratins. The mechanical properties of these cells were analyzed by atomic force microscopy (AFM) and magnetic tweezers experiments. We found a strong and highly significant softening of keratin-deficient keratinocytes when analyzed by AFM on the cell body and above the nucleus. Magnetic tweezers experiments fully confirmed these results showing, in addition, high viscous contributions to magnetic bead displacement in keratin-lacking cells. Keratin loss neither affected actin or microtubule networks nor their overall protein concentration. Furthermore, depolymerization of actin preserves cell softening in the absence of keratin. On reexpression of the sole basal epidermal keratin pair K5/14, the keratin filament network was reestablished, and mechanical properties were restored almost to WT levels in both experimental setups. The data presented here demonstrate the importance of keratin filaments for mechanical resilience of keratinocytes and indicate that expression of a single keratin pair is sufficient for almost complete reconstitution of their mechanical properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
An intact keratin network is crucial for mechanical integrity and barrier function in keratinocyte cell sheets.Cell Mol Life Sci. 2020 Nov;77(21):4397-4411. doi: 10.1007/s00018-019-03424-7. Epub 2020 Jan 7. Cell Mol Life Sci. 2020. PMID: 31912195 Free PMC article.
-
In vitro characteristics of early epidermal progenitors isolated from keratin 14 (K14)-deficient mice: insights into the role of keratin 17 in mouse keratinocytes.J Cell Physiol. 1999 Sep;180(3):409-21. doi: 10.1002/(SICI)1097-4652(199909)180:3<409::AID-JCP12>3.0.CO;2-V. J Cell Physiol. 1999. PMID: 10430181
-
Keratins significantly contribute to cell stiffness and impact invasive behavior.Proc Natl Acad Sci U S A. 2013 Nov 12;110(46):18507-12. doi: 10.1073/pnas.1310493110. Epub 2013 Oct 28. Proc Natl Acad Sci U S A. 2013. PMID: 24167274 Free PMC article.
-
Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia.J Anat. 2009 Apr;214(4):516-59. doi: 10.1111/j.1469-7580.2009.01066.x. J Anat. 2009. PMID: 19422428 Free PMC article. Review.
-
Keratins: a structural scaffold with emerging functions.Cell Mol Life Sci. 2003 Jan;60(1):56-71. doi: 10.1007/s000180300004. Cell Mol Life Sci. 2003. PMID: 12613658 Free PMC article. Review.
Cited by
-
Mouse Keratinocytes Without Keratin Intermediate Filaments Demonstrate Substrate Stiffness Dependent Behaviors.Cell Mol Bioeng. 2018 May 2;11(3):163-174. doi: 10.1007/s12195-018-0526-y. eCollection 2018 Jun. Cell Mol Bioeng. 2018. PMID: 31719883 Free PMC article.
-
Mechanotransduction in Skin Inflammation.Cells. 2022 Jun 25;11(13):2026. doi: 10.3390/cells11132026. Cells. 2022. PMID: 35805110 Free PMC article. Review.
-
Junctional epithelium and hemidesmosomes: Tape and rivets for solving the "percutaneous device dilemma" in dental and other permanent implants.Bioact Mater. 2022 Mar 19;18:178-198. doi: 10.1016/j.bioactmat.2022.03.019. eCollection 2022 Dec. Bioact Mater. 2022. PMID: 35387164 Free PMC article. Review.
-
Atomic Force Microscopy Provides New Mechanistic Insights into the Pathogenesis of Pemphigus.Front Immunol. 2018 Mar 28;9:485. doi: 10.3389/fimmu.2018.00485. eCollection 2018. Front Immunol. 2018. PMID: 29643851 Free PMC article. Review.
-
Keratin Retraction and Desmoglein3 Internalization Independently Contribute to Autoantibody-Induced Cell Dissociation in Pemphigus Vulgaris.Front Immunol. 2018 Apr 25;9:858. doi: 10.3389/fimmu.2018.00858. eCollection 2018. Front Immunol. 2018. PMID: 29922278 Free PMC article.
References
-
- Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–153. - PubMed
-
- Kasza KE, et al. The cell as a material. Curr Opin Cell Biol. 2007;19(1):101–107. - PubMed
-
- Beil M, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5(9):803–811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

