Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia
- PMID: 24167258
- PMCID: PMC3831977
- DOI: 10.1073/pnas.1318257110
Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia
Erratum in
- Proc Natl Acad Sci U S A. 2014 Sep 23;111(38):14002
Abstract
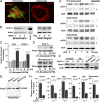
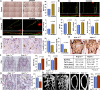
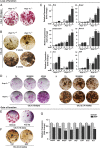
Although hyponatremia is known to be associated with osteoporosis and a high fracture risk, the mechanism through which bone loss ensues has remained unclear. As hyponatremic patients have elevated circulating arginine-vasopressin (AVP) levels, we examined whether AVP can affect the skeleton directly as yet another component of the pituitary-bone axis. Here, we report that the two Avp receptors, Avpr1α and Avpr2, coupled to Erk activation, are expressed in osteoblasts and osteoclasts. AVP injected into wild-type mice enhanced and reduced, respectively, the formation of bone-resorbing osteoclasts and bone-forming osteoblasts. Conversely, the exposure of osteoblast precursors to Avpr1α or Avpr2 antagonists, namely SR49059 or ADAM, increased osteoblastogenesis, as did the genetic deletion of Avpr1α. In contrast, osteoclast formation and bone resorption were both reduced in Avpr1α(-/-) cultures. This process increased bone formation and reduced resorption resulted in a profound enhancement of bone mass in Avpr1α(-/-) mice and in wild-type mice injected with SR49059. Collectively, the data not only establish a primary role for Avp signaling in bone mass regulation, but also call for further studies on the skeletal actions of Avpr inhibitors used commonly in hyponatremic patients.
Keywords: osteopenia; oxytocin; pituitary hormone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Abe E, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115(2):151–162. - PubMed
-
- Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–260. - PubMed
-
- Zaidi M, et al. Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism. 2013. The pituitary-bone connection. 8th ed. eds Rosen CJ, Bouillon R, Compston JE, Rosen V (Wiley-Blackwell, Hoboken, NJ), pp 969–977.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

