Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ
- PMID: 24167275
- PMCID: PMC3832005
- DOI: 10.1073/pnas.1318972110
Cyclic diguanosine monophosphate represses bacterial flagella synthesis by interacting with the Walker A motif of the enhancer-binding protein FleQ
Abstract
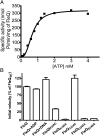
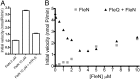
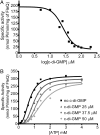
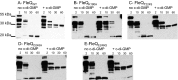
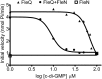
The transcription factor FleQ is a bacterial AAA+ ATPase enhancer-binding protein that is the master activator of flagella gene expression in the opportunistic bacterial pathogen Pseudomonas aeruginosa. Homologs of FleQ are present in all Pseudomonas species and in many polarly flagellated gamma proteobacteria. Cyclic diguanosine monophosphate (c-di-GMP) is a second messenger that controls the transition between planktonic and biofilm modes of growth in bacteria in response to diverse environmental signals. C-di-GMP binds to FleQ to dampen its activity, causing down-regulation of flagella gene expression. This action is potentiated in the simultaneous presence of another protein, FleN. We explored the effect of c-di-GMP and FleN on the ATPase activity of FleQ and found that a relatively low concentration of c-di-GMP competitively inhibited FleQ ATPase activity, suggesting that c-di-GMP competes with ATP for binding to the Walker A motif of FleQ. Confirming this, a FleQ Walker A motif mutant failed to bind c-di-GMP. FleN, whose gene is regulated by FleQ, also inhibited FleQ ATPase activity, and FleQ ATPase activity was much more inhibited by c-di-GMP in the presence of FleN than in its absence. These results indicate that FleN and c-di-GMP cooperate to inhibit FleQ activity and, by extension, flagella synthesis in P. aeruginosa. The Walker A motif of FleQ is perfectly conserved, opening up the possibility that other AAA+ ATPases may respond to c-di-GMP.
Keywords: AAA+ protein; competitive inhibition; transcription factor; σ54.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mechanistic insights into c-di-GMP-dependent control of the biofilm regulator FleQ from Pseudomonas aeruginosa.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):E209-18. doi: 10.1073/pnas.1523148113. Epub 2015 Dec 28. Proc Natl Acad Sci U S A. 2016. PMID: 26712005 Free PMC article.
-
The FleQ protein from Pseudomonas aeruginosa functions as both a repressor and an activator to control gene expression from the pel operon promoter in response to c-di-GMP.Nucleic Acids Res. 2012 Aug;40(15):7207-18. doi: 10.1093/nar/gks384. Epub 2012 May 11. Nucleic Acids Res. 2012. PMID: 22581773 Free PMC article.
-
Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor.Mol Microbiol. 2008 Jul;69(2):376-89. doi: 10.1111/j.1365-2958.2008.06281.x. Mol Microbiol. 2008. PMID: 18485075 Free PMC article.
-
Flip the switch: the role of FleQ in modulating the transition between the free-living and sessile mode of growth in Pseudomonas aeruginosa.J Bacteriol. 2024 Mar 21;206(3):e0036523. doi: 10.1128/jb.00365-23. Epub 2024 Mar 4. J Bacteriol. 2024. PMID: 38436566 Free PMC article. Review.
-
Taming the flagellar motor of pseudomonads with a nucleotide messenger.Environ Microbiol. 2020 Jul;22(7):2496-2513. doi: 10.1111/1462-2920.15036. Epub 2020 May 5. Environ Microbiol. 2020. PMID: 32329141 Review.
Cited by
-
Diversity of Cyclic Di-GMP-Binding Proteins and Mechanisms.J Bacteriol. 2016 Jan 1;198(1):32-46. doi: 10.1128/JB.00333-15. J Bacteriol. 2016. PMID: 26055114 Free PMC article. Review.
-
A Family of Small Intrinsically Disordered Proteins Involved in Flagellum-Dependent Motility in Salmonella enterica.J Bacteriol. 2018 Dec 20;201(2):e00415-18. doi: 10.1128/JB.00415-18. Print 2019 Jan 15. J Bacteriol. 2018. PMID: 30373755 Free PMC article.
-
Formation, Development, and Cross-Species Interactions in Biofilms.Front Microbiol. 2022 Jan 4;12:757327. doi: 10.3389/fmicb.2021.757327. eCollection 2021. Front Microbiol. 2022. PMID: 35058893 Free PMC article. Review.
-
Bacterial-fungal metabolic interactions within the microbiota and their potential relevance in human health and disease: a short review.Gut Microbes. 2022 Jan-Dec;14(1):2105610. doi: 10.1080/19490976.2022.2105610. Gut Microbes. 2022. PMID: 35903007 Free PMC article. Review.
-
OsaR (PA0056) Functions as a Repressor of the Gene fleQ Encoding an Important Motility Regulator in Pseudomonas aeruginosa.J Bacteriol. 2021 Sep 23;203(20):e0014521. doi: 10.1128/JB.00145-21. Epub 2021 Aug 2. J Bacteriol. 2021. PMID: 34339300 Free PMC article.
References
-
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7(4):263–273. - PubMed
-
- Jenal U, Malone J. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu Rev Genet. 2006;40:385–407. - PubMed
-
- Mills E, Pultz IS, Kulasekara HD, Miller SI. The bacterial second messenger c-di-GMP: Mechanisms of signalling. Cell Microbiol. 2011;13(8):1122–1129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

