Targeting peripheral opioid receptors to promote analgesic and anti-inflammatory actions
- PMID: 24167491
- PMCID: PMC3807052
- DOI: 10.3389/fphar.2013.00132
Targeting peripheral opioid receptors to promote analgesic and anti-inflammatory actions
Abstract
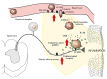
Mechanisms of endogenous pain control are significant. Increasing studies have clearly produced evidence for the clinical usefulness of opioids in peripheral analgesia. The immune system uses mechanisms of cell migration not only to fight pathogens but also to control pain and inflammation within injured tissue. It has been demonstrated that peripheral inflammatory pain can be effectively controlled by an interaction of immune cell-derived opioid peptides with opioid receptors on peripheral sensory nerve terminals. Experimental and clinical studies have clearly shown that activation of peripheral opioid receptors with exogenous opioid agonists and endogenous opioid peptides are able to produce significant analgesic and anti-inflammatory effects, without central opioid mediated side effects (e.g., respiratory depression, sedation, tolerance, dependence). This article will focus on the role of opioids in peripheral inflammatory conditions and the clinical implications of targeting peripheral opioid receptors.
Keywords: analgesia; anti-inflammatory; immune cells; inflammation; opioids; pain; peripheral opioid receptors.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

