How the prohormone theory solved two important controversies in hormonal and neural Peptide biosynthesis
- PMID: 24167501
- PMCID: PMC3805937
- DOI: 10.3389/fendo.2013.00148
How the prohormone theory solved two important controversies in hormonal and neural Peptide biosynthesis
Abstract
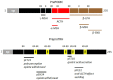
This Prohormone Theory was simultaneously proposed in 1967 by two independent groups using two different approaches and two experimental models. Donald Steiner, in elegant pulse-chase experiments, proposed the existence of proinsulin when he observed that a human insulinoma was producing higher MW forms of immunoreactive insulin, subsequently transformed into insulin-like material (1). Simultaneously and independently, Michel Chrétien, based on amino acid sequence homologies between three pituitary peptides, β-lipotropic hormone (β-LPH), γ-LPH, and β-melanocyte-stimulating hormone (β-MSH), concluded that active peptide hormones are derived from endoproteolytic cleavages of inactive precursors, apparently at pairs of basic amino acids (2). One year later, Donald Chance confirmed that the cleavage sites in proinsulin were also made of paired basic amino acids (3). This novel paradigm solved two major controversies on the biosynthesis of both insulin and neuropeptides. This short review describes how.
Keywords: biosynthesis; neuropeptides; peptide hormones; prohormone theory; proprotein convertases.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

