Methylglyoxal evokes pain by stimulating TRPA1
- PMID: 24167592
- PMCID: PMC3805573
- DOI: 10.1371/journal.pone.0077986
Methylglyoxal evokes pain by stimulating TRPA1
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/e707d50a-13b3-4cc3-b507-7d8360d8f048
Abstract
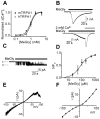
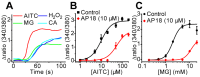
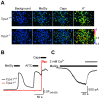
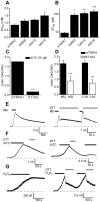
Diabetic neuropathy is a severe complication of long-standing diabetes and one of the major etiologies of neuropathic pain. Diabetes is associated with an increased formation of reactive oxygen species and the electrophilic dicarbonyl compound methylglyoxal (MG). Here we show that MG stimulates heterologously expressed TRPA1 in CHO cells and natively expressed TRPA1 in MDCK cells and DRG neurons. MG evokes [Ca(2+)]i-responses in TRPA1 expressing DRG neurons but is without effect in neurons cultured from Trpa1(-/-) mice. Consistent with a direct, intracellular action, we show that methylglyoxal is significantly more potent as a TRPA1 agonist when applied to the intracellular face of excised membrane patches than to intact cells. Local intraplantar administration of MG evokes a pain response in Trpa1(+/+) but not in Trpa1(-/-) mice. Furthermore, persistently increased MG levels achieved by two weeks pharmacological inhibition of glyoxalase-1 (GLO-1), the rate-limiting enzyme responsible for detoxification of MG, evokes a progressive and marked thermal (cold and heat) and mechanical hypersensitivity in wildtype but not in Trpa1(-/-) mice. Our results thus demonstrate that TRPA1 is required both for the acute pain response evoked by topical MG and for the long-lasting pronociceptive effects associated with elevated MG in vivo. In contrast to our observations in DRG neurons, MG evokes indistinguishable [Ca(2+)]i-responses in pancreatic β-cells cultured from Trpa1(+/+) and Trpa1(-/-) mice. In vivo, the TRPA1 antagonist HC030031 impairs glucose clearance in the glucose tolerance test both in Trpa1(+/+) and Trpa1(-/-) mice, indicating a non-TRPA1 mediated effect and suggesting that results obtained with this compound should be interpreted with caution. Our results show that TRPA1 is the principal target for MG in sensory neurons but not in pancreatic β-cells and that activation of TRPA1 by MG produces a painful neuropathy with the behavioral hallmarks of diabetic neuropathy.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

