The effect of sugar-free versus sugar-sweetened beverages on satiety, liking and wanting: an 18 month randomized double-blind trial in children
- PMID: 24167595
- PMCID: PMC3805601
- DOI: 10.1371/journal.pone.0078039
The effect of sugar-free versus sugar-sweetened beverages on satiety, liking and wanting: an 18 month randomized double-blind trial in children
Abstract
Background: Substituting sugar-free for sugar-sweetened beverages reduces weight gain. A possible explanation is that sugar-containing and sugar-free beverages cause the same degree of satiety. However, this has not been tested in long-term trials.
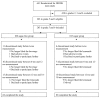
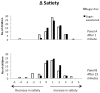
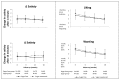
Methods: We randomized 203 children aged 7-11 years to receive 250 mL per day of an artificially sweetened sugar-free beverage or a similarly looking and tasting sugar-sweetened beverage. We measured satiety on a 5-point scale by questionnaire at 0, 6, 12 and 18 months. We calculated the change in satiety from before intake to 1 minute after intake and 15 minutes after intake. We then calculated the odds ratio that satiety increased by 1 point in the sugar-group versus the sugar-free group. We also investigated how much the children liked and wanted the beverages.
Results: 146 children or 72% completed the study. We found no statistically significant difference in satiety between the sugar-free and sugar-sweetened group; the adjusted odds ratio for a 1 point increase in satiety in the sugar group versus the sugar-free group was 0.77 at 1 minute (95% confidence interval, 0.46 to 1.29), and 1.44 at 15 minutes after intake (95% CI, 0.86 to 2.40). The sugar-group liked and wanted their beverage slightly more than the sugar-free group, adjusted odds ratio 1.63 (95% CI 1.05 to 2.54) and 1.65 (95% CI 1.07 to 2.55), respectively.
Conclusions: Sugar-sweetened and sugar-free beverages produced similar satiety. Therefore when children are given sugar-free instead of sugar-containing drinks they might not make up the missing calories from other sources. This may explain our previous observation that children in the sugar-free group accumulated less body fat than those in the sugar group.
Trial registration: ClinicalTrials.gov NCT00893529 http://clinicaltrials.gov/show/NCT00893529.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

