Evidence for a retroviral insertion in TRPM1 as the cause of congenital stationary night blindness and leopard complex spotting in the horse
- PMID: 24167615
- PMCID: PMC3805535
- DOI: 10.1371/journal.pone.0078280
Evidence for a retroviral insertion in TRPM1 as the cause of congenital stationary night blindness and leopard complex spotting in the horse
Abstract
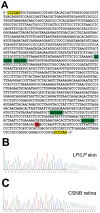
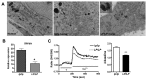
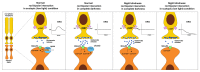
Leopard complex spotting is a group of white spotting patterns in horses caused by an incompletely dominant gene (LP) where homozygotes (LP/LP) are also affected with congenital stationary night blindness. Previous studies implicated Transient Receptor Potential Cation Channel, Subfamily M, Member 1 (TRPM1) as the best candidate gene for both CSNB and LP. RNA-Seq data pinpointed a 1378 bp insertion in intron 1 of TRPM1 as the potential cause. This insertion, a long terminal repeat (LTR) of an endogenous retrovirus, was completely associated with LP, testing 511 horses (χ(2)=1022.00, p<<0.0005), and CSNB, testing 43 horses (χ(2)=43, p<<0.0005). The LTR was shown to disrupt TRPM1 transcription by premature poly-adenylation. Furthermore, while deleterious transposable element insertions should be quickly selected against the identification of this insertion in three ancient DNA samples suggests it has been maintained in the horse gene pool for at least 17,000 years. This study represents the first description of an LTR insertion being associated with both a pigmentation phenotype and an eye disorder.
Conflict of interest statement
Figures