Cdc48: a swiss army knife of cell biology
- PMID: 24167726
- PMCID: PMC3791797
- DOI: 10.1155/2013/183421
Cdc48: a swiss army knife of cell biology
Abstract
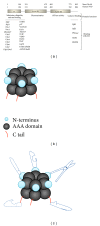
Cdc48 (also called VCP and p97) is an abundant protein that plays essential regulatory functions in a broad array of cellular processes. Working with various cofactors, Cdc48 utilizes its ATPase activity to promote the assembly and disassembly of protein complexes. Here, we review key biological functions and regulation of Cdc48 in ubiquitin-related events. Given the broad employment of Cdc48 in cell biology and its intimate ties to human diseases (e.g., amyotrophic lateral sclerosis), studies of Cdc48 will bring significant insights into the mechanism and function of ubiquitin in health and diseases.
Figures

References
-
- Jentsch S, Rumpf S. Cdc48 (p97): a “molecular gearbox” in the ubiquitin pathway? Trends in Biochemical Sciences. 2007;32(1):6–11. - PubMed
-
- Dantuma NP, Hoppe T. Growing sphere of influence: Cdc48/p97 orchestrates ubiquitin-dependent extraction from chromatin. Trends in Cell Biology. 2012;22(9):483–491. - PubMed
-
- Dargemont C, Ossareh-Nazari B. Cdc48/p97, a key actor in the interplay between autophagy and ubiquitin/proteasome catabolic pathways. Biochimica et Biophysica Acta. 2012;1823(1):138–144. - PubMed
-
- Wolf DH, Stolz A. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochimica et Biophysica Acta. 2012;1823(1):117–124. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

