Effect of mushroom diet on pharmacokinetics of gabapentin in healthy Chinese subjects
- PMID: 24168107
- PMCID: PMC4168387
- DOI: 10.1111/bcp.12273
Effect of mushroom diet on pharmacokinetics of gabapentin in healthy Chinese subjects
Abstract
Aims: This study evaluated the pharmacokinetics of gabapentin in Chinese subjects who received a diet rich in shiitake mushrooms. Shiitake mushrooms have been shown to contain high amount of ergothioneine. In vitro studies have shown that OCTN1-mediated secretion of gabapentin is trans-stimulated by ergothioneine. This study also investigated the concentrations of ergothioneine in plasma at baseline and following mushroom consumption.
Methods: Ten healthy male subjects were recruited and received a diet containing no mushrooms (treatment A) or a high mushroom diet (treatment B; after at least a 7 day washout period) 1 day prior to administration of a single oral dose of gabapentin 600 mg.
Results: Ingestion of shiitake mushrooms produced significant increases in plasma ergothioneine concentrations that were sustained for more than 48 h. A statistically significant but modest increase in the renal clearance (CLR ) of gabapentin occurred after intake of the mushroom diet (91.1 ± 25.1 vs. 76.9 ± 20.6 ml min(-1) , P = 0.031). No significant changes in AUC(0,tlast ) of gabapentin were observed (P = 0.726). Creatinine clearance did not correlate with CLR of gabapentin at baseline (treatment A). After ingestion of the mushroom diet, creatinine clearance accounted for 65.3% of the variance in CLR of gabapentin.
Conclusions: These data suggest that diet-drug pharmacokinetic interactions may occur during co-exposure to gabapentin and mushroom constituents. However, as it does not affect the AUC(0,tlast ) of gabapentin, it may not have clinically important consequences. Shiitake mushrooms can also be used as a source of ergothioneine for future clinical studies.
Keywords: ergothioneine; gabapentin; mushroom; novel human organic cation transporter 1; pharmacokinetics.
© 2013 The British Pharmacological Society.
Figures
References
-
- Bar AV. Gabapentin for the treatment of cancer-related pain syndromes. Rev Recent Clin Trials. 2010;5:174–178. - PubMed
-
- Beydoun A, Uthman BM, Sackellares JC. Gabapentin: pharmacokinetics, efficacy, and safety. Clin Neuropharmacol. 1995;18:469–481. - PubMed
-
- Urban TJ, Brown C, Castro RA, Shah N, Mercer R, Huang Y, Brett CM, Burchard EG, Giacomini KM. Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther. 2008;83:416–421. - PubMed
-
- Urban TJ, Yang C, Lagpacan LL, Brown C, Castro RA, Taylor TR, Huang CC, Stryke D, Johns SJ, Kawamoto M, Carlson EJ, Ferrin TE, Burchard EG, Giacomini KM. Functional effects of protein sequence polymorphisms in the organic cation/ergothioneine transporter OCTN1 (SLC22A4) Pharmacogenet Genomics. 2007;17:773–782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

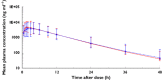
 , treatment A;
, treatment A;  …
… 