Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration
- PMID: 24168314
- PMCID: PMC3938917
- DOI: 10.1089/ten.TEA.2013.0111
Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration
Abstract
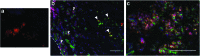
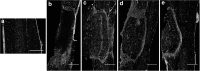
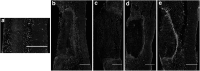
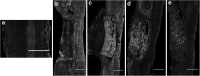
Spinal cord injury (SCI) results in loss of sensory and motor function below the level of injury and has limited available therapies. The host response to SCI is typified by limited endogenous repair, and biomaterial bridges offer the potential to alter the microenvironment to promote regeneration. Porous multiple channel bridges implanted into the injury provide stability to limit secondary damage and support cell infiltration that limits cavity formation. At the same time, the channels provide a path that physically directs axon growth across the injury. Using a rat spinal cord hemisection injury model, we investigated the dynamics of axon growth, myelination, and scar formation within and around the bridge in vivo for 6 months, at which time the bridge has fully degraded. Axons grew into and through the channels, and the density increased overtime, resulting in the greatest axon density at 6 months postimplantation, despite complete degradation of the bridge by that time point. Furthermore, the persistence of these axons contrasts with reports of axonal dieback in other models and is consistent with axon stability resulting from some degree of connectivity. Immunostaining of axons revealed both motor and sensory origins of the axons found in the channels of the bridge. Extensive myelination was observed throughout the bridge at 6 months, with centrally located and peripheral channels seemingly myelinated by oligodendrocytes and Schwann cells, respectively. Chondroitin sulfate proteoglycan deposition was restricted to the edges of the bridge, was greatest at 1 week, and significantly decreased by 6 weeks. The dynamics of collagen I and IV, laminin, and fibronectin deposition varied with time. These studies demonstrate that the bridge structure can support substantial long-term axon growth and myelination with limited scar formation.
Figures




References
-
- Richardson P.M., McGuinness U.M., and Aguayo A.J.Axons from CNS neurons regenerate into PNS grafts. Nature 284,264, 1980 - PubMed
-
- David S., and Aguayo A.J.Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214,931, 1981 - PubMed
-
- Geller H.M., and Fawcett J.W.Building a bridge: engineering spinal cord repair. Exp Neurol 174,125, 2002 - PubMed
-
- Wang M., Zhai P., Chen X., Schreyer D.J., Sun X., and Cui F.Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev 17,177, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

