Improving activity and enantioselectivity of lipase via immobilization on macroporous resin for resolution of racemic 1- phenylethanol in non-aqueous medium
- PMID: 24168516
- PMCID: PMC4228463
- DOI: 10.1186/1472-6750-13-92
Improving activity and enantioselectivity of lipase via immobilization on macroporous resin for resolution of racemic 1- phenylethanol in non-aqueous medium
Abstract
Background: Burkholderia cepacia lipase (BCL) has been proved to be capable of resolution reactions. However, its free form usually exhibits low stability, bad resistance and no reusability, which restrict its further industrial applications. Therefore, it is of great importance to improve the catalytic performance of free lipase in non-aqueous medium.
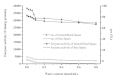
Results: In this work, macroporous resin NKA (MPR-NKA) was utilized as support for lipase immobilization. Racemic transesterification of 1-phenylethanol with vinyl acetate was chosen as model reaction. Compared with its free form, the enzyme activity and enantioselectivity (ees) of the immobilized lipase have been significantly enhanced. The immobilized BCL exhibited a satisfactory thermostability over a wide range of temperature (from 10 to 65°C) and an excellent catalytic efficiency. After being used for more than 30 successive batches, the immobilized lipase still kept most of its activity. In comparison with other immobilized lipases, the immobilized BCL also exhibits better catalytic efficiency, which indicates a significant potential in industrial applications.
Conclusion: The results of this study have proved that MPR-NKA was an excellent support for immobilization of lipase via the methods of N2 adsorption-desorption, scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) and Fourier transform-infrared spectroscopy (FT-IR). The improvement of enzyme activity and ees for the immobilized lipase was closely correlated with the alteration of its secondary structure. This information may contribute to a better understanding of the mechanism of immobilization and enzymatic biotransformation in non-aqueous medium.
Figures









References
-
- Liu T, Liu Y, Wang X, Li Q, Wang J, Yan Y. Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA. J Mol Catal B: Enzym. 2011;71(1–2):45–50.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

