Fluorogenic, two-photon-triggered photoclick chemistry in live mammalian cells
- PMID: 24168622
- PMCID: PMC3866799
- DOI: 10.1021/ja407867a
Fluorogenic, two-photon-triggered photoclick chemistry in live mammalian cells
Abstract
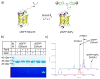
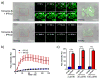
The tetrazole-based photoclick chemistry has provided a powerful tool to image proteins in live cells. To extend photoclick chemistry to living organisms with improved spatiotemporal control, here we report the design of naphthalene-based tetrazoles that can be efficiently activated by two-photon excitation with a 700 nm femtosecond pulsed laser. A water-soluble, cell-permeable naphthalene-based tetrazole was identified that reacts with acrylamide with the effective two-photon cross-section for the cycloaddition reaction determined to be 3.8 GM. Furthermore, the use of this naphthalene-tetrazole for real-time, spatially controlled imaging of microtubules in live mammalian cells via the fluorogenic, two-photon-triggered photoclick chemistry was demonstrated.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

