MiR-125b acts as an oncogene in glioblastoma cells and inhibits cell apoptosis through p53 and p38MAPK-independent pathways
- PMID: 24169356
- PMCID: PMC3844918
- DOI: 10.1038/bjc.2013.672
MiR-125b acts as an oncogene in glioblastoma cells and inhibits cell apoptosis through p53 and p38MAPK-independent pathways
Abstract
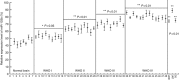
Background: We have recently identified miR-125b upregulation in glioblastoma (GMB). The aim of this study is to determine the correlation between miR-125b expression and malignant grades of glioma and the genes targeted by miR-125b.
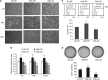
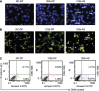
Methods: Real-time PCR was employed to measure the expression level of miR-125b. Cell viability was evaluated by cell growth and colony formation in soft-agar assays. Cell apoptosis was determined by Hoechst 33342 staining and AnnexinV-FITC assay. The Luciferase assay was used to confirm the actual binding sites of p38MAPK mRNA. Western blot was used to detect the gene expression level.
Results: The expression level of miR-125b is positively correlated with the malignant grade of glioma. Ectopic expression of miR-125b promotes the proliferation of GMB cells. Knockdown of endogenous miR-125b inhibits cell proliferation and promotes cell apoptosis. Further studies reveal that p53 is regulated by miR-125b. However, downregulation of the endogenous miR-125b also results in p53-independent apoptotic pathway leading to apoptosis in p53 mutated U251 cells and p53 knockdown U87 cells. Moreover, p38MAPK is also regulated by miR-125b and downregulation of miR-125b activates the p38MAPK-induced mitochondria apoptotic pathway.
Conclusion: High-level expression of miR-125b is associated with poor outcomes of GMB. MiR-125b may have an oncogenic role in GMB cells by promoting cell proliferation and inhibiting apoptosis.
Figures

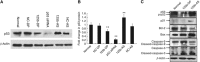

Similar articles
-
miR-424 induces apoptosis in glioblastoma cells and targets AKT1 and RAF1 oncogenes from the ERBB signaling pathway.Eur J Pharmacol. 2021 Sep 5;906:174273. doi: 10.1016/j.ejphar.2021.174273. Epub 2021 Jun 18. Eur J Pharmacol. 2021. PMID: 34153339
-
MicroRNA-125b mimic inhibits ischemia reperfusion-induced neuroinflammation and aberrant p53 apoptotic signalling activation through targeting TP53INP1.Brain Behav Immun. 2018 Nov;74:154-165. doi: 10.1016/j.bbi.2018.09.002. Epub 2018 Sep 5. Brain Behav Immun. 2018. PMID: 30193876
-
Potential contribution of microRNA-125b targeting p38MAPK to relieving intermittent hypoxia-induced dementia of rat models.J Clin Neurosci. 2019 Jun;64:234-241. doi: 10.1016/j.jocn.2019.03.002. Epub 2019 Apr 15. J Clin Neurosci. 2019. PMID: 31000329
-
Oncomir miR-125b suppresses p14(ARF) to modulate p53-dependent and p53-independent apoptosis in prostate cancer.PLoS One. 2013 Apr 9;8(4):e61064. doi: 10.1371/journal.pone.0061064. Print 2013. PLoS One. 2013. PMID: 23585871 Free PMC article.
-
Long noncoding RNA LINC00152 promotes cell proliferation through competitively binding endogenous miR-125b with MCL-1 by regulating mitochondrial apoptosis pathways in ovarian cancer.Cancer Med. 2018 Sep;7(9):4530-4541. doi: 10.1002/cam4.1547. Epub 2018 Jul 20. Cancer Med. 2018. PMID: 30030896 Free PMC article.
Cited by
-
Knocking down of LINC01220 inhibits proliferation and induces apoptosis of endometrial carcinoma through silencing MAPK11.Biosci Rep. 2019 Jul 25;39(7):BSR20181794. doi: 10.1042/BSR20181794. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31123170 Free PMC article.
-
miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine.Noncoding RNA. 2024 Feb 21;10(2):16. doi: 10.3390/ncrna10020016. Noncoding RNA. 2024. PMID: 38525735 Free PMC article. Review.
-
Non-Coding RNAs and Brain Tumors: Insights Into Their Roles in Apoptosis.Front Cell Dev Biol. 2022 Jan 17;9:792185. doi: 10.3389/fcell.2021.792185. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35111757 Free PMC article. Review.
-
Epigenetic Underpinnings of Inflammation: A Key to Unlock the Tumor Microenvironment in Glioblastoma.Front Immunol. 2022 Apr 29;13:869307. doi: 10.3389/fimmu.2022.869307. eCollection 2022. Front Immunol. 2022. PMID: 35572545 Free PMC article.
-
Differential miRNA Expression Profiling Reveals Correlation of miR125b-5p with Persistent Infection of Japanese Encephalitis Virus.Int J Mol Sci. 2021 Apr 19;22(8):4218. doi: 10.3390/ijms22084218. Int J Mol Sci. 2021. PMID: 33921710 Free PMC article.
References
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. - PubMed
-
- Gefen N, Binder V, Zaliova M, Linka Y, Morrow M, Novosel A, Edry L, Hertzberg L, Shomron N, Williams O, Trka J, Borkhardt A, Izraeli S. Hsa-mir-125b-2 is highly expressed in childhood ETV6/RUNX1 (TEL/AML1) leukemias and confers survival advantage to growth inhibitory signals independent of p53. Leukemia. 2010;24 (1:89–96. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

