Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining
- PMID: 24169803
- PMCID: PMC3957101
- DOI: 10.1038/npp.2013.307
Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining
Abstract
Methamphetamine is a widely abused illicit drug. Recent epidemiological studies showed that methamphetamine increases the risk for developing Parkinson's disease (PD) in agreement with animal studies showing dopaminergic neurotoxicity. We examined the effect of repeated low and medium doses vs single high dose of methamphetamine on degeneration of dopaminergic terminals and cell bodies. Mice were given methamphetamine in one of the following paradigms: three injections of 5 or 10 mg/kg at 3 h intervals or a single 30 mg/kg injection. The integrity of dopaminergic fibers and cell bodies was assessed at different time points after methamphetamine by tyrosine hydroxylase immunohistochemistry and silver staining. The 3 × 10 protocol yielded the highest loss of striatal dopaminergic terminals, followed by the 3 × 5 and 1 × 30. Some degenerating axons could be followed from the striatum to the substantia nigra pars compacta (SNpc). All protocols induced similar significant degeneration of dopaminergic neurons in the SNpc, evidenced by amino-cupric-silver-stained dopaminergic neurons. These neurons died by necrosis and apoptosis. Methamphetamine also killed striatal neurons. By using D1-Tmt/D2-GFP BAC transgenic mice, we observed that degenerating striatal neurons were equally distributed between direct and indirect medium spiny neurons. Despite the reduced number of dopaminergic neurons in the SNpc at 30 days after treatment, there was a partial time-dependent recovery of dopamine terminals beginning 3 days after treatment. Locomotor activity and motor coordination were robustly decreased 1-3 days after treatment, but recovered at later times along with dopaminergic terminals. These data provide direct evidence that methamphetamine causes long-lasting loss/degeneration of dopaminergic cell bodies in the SNpc, along with destruction of dopaminergic terminals in the striatum.
Figures

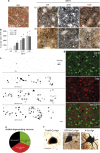


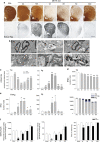
References
-
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. - PubMed
-
- Ares-Santos S, Granado N, Moratalla R.2013Neurobiology of methamphetamineIn: Miller PM, (ed)Biological Research on Addiction: Comprehensive Addictive Behaviors and DisordersChapter 57.Elsevier, Academic Press: San Diego, CA, USA; 579–591.ISBN: 9780123983350.
-
- Ares-Santos S, Granado N, Moratalla R.2013Role of dopamine receptors in the neurotoxicity of methamphetamine J Intern Med 273437–453.(review). - PubMed
-
- Ares-Santos S, Granado N, Oliva I, O'Shea E, Martin ED, Colado MI, et al. Dopamine D(1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis. 2012;45:810–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

