Non-publication of large randomized clinical trials: cross sectional analysis
- PMID: 24169943
- PMCID: PMC3812466
- DOI: 10.1136/bmj.f6104
Non-publication of large randomized clinical trials: cross sectional analysis
Abstract
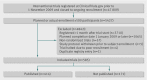
Objective: To estimate the frequency with which results of large randomized clinical trials registered with ClinicalTrials.gov are not available to the public.
Design: Cross sectional analysis
Setting: Trials with at least 500 participants that were prospectively registered with ClinicalTrials.gov and completed prior to January 2009.
Data sources: PubMed, Google Scholar, and Embase were searched to identify published manuscripts containing trial results. The final literature search occurred in November 2012. Registry entries for unpublished trials were reviewed to determine whether results for these studies were available in the ClinicalTrials.gov results database.
Main outcome measures: The frequency of non-publication of trial results and, among unpublished studies, the frequency with which results are unavailable in the ClinicalTrials.gov database.
Results: Of 585 registered trials, 171 (29%) remained unpublished. These 171 unpublished trials had an estimated total enrollment of 299,763 study participants. The median time between study completion and the final literature search was 60 months for unpublished trials. Non-publication was more common among trials that received industry funding (150/468, 32%) than those that did not (21/117, 18%), P=0.003. Of the 171 unpublished trials, 133 (78%) had no results available in ClinicalTrials.gov.
Conclusions: Among this group of large clinical trials, non-publication of results was common and the availability of results in the ClinicalTrials.gov database was limited. A substantial number of study participants were exposed to the risks of trial participation without the societal benefits that accompany the dissemination of trial results.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Effect of funding source on publication bias is not so clear cut.BMJ. 2013 Dec 23;347:f7582. doi: 10.1136/bmj.f7582. BMJ. 2013. PMID: 24366203 No abstract available.
-
Working together to improve the credibility and transparency of clinical research.BMJ. 2013 Dec 23;347:f7590. doi: 10.1136/bmj.f7590. BMJ. 2013. PMID: 24366654 No abstract available.
-
Authors' reply to van Lent and Out.BMJ. 2013 Dec 23;347:f7588. doi: 10.1136/bmj.f7588. BMJ. 2013. PMID: 24366677 No abstract available.
References
-
- Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA 2000;283:2701-11. - PubMed
-
- Moreno J, Caplan AL, Wolpe PR. Updating protections for human subjects involved in research. Project on Informed Consent, Human Research Ethics Group. JAMA 1998;280:1951-8. - PubMed
-
- Food and Drug Administration Modernization Act of 1997. US Public Law 105-15. (1997, Nov 21); 21 USC 301.
-
- De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med 2004;351:1250-1. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources