Bone cell communication factors and Semaphorins
- PMID: 24171101
- PMCID: PMC3810552
- DOI: 10.1038/bonekey.2012.183
Bone cell communication factors and Semaphorins
Abstract
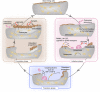
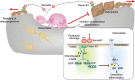
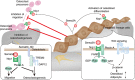
Bone tissue is continuously renewed throughout adult life by a process called 'remodeling', which involves a dynamic interplay among bone cells including osteoclasts, osteoblasts and osteocytes. For example, a tight coupling between bone resorption and formation is essential for the homeostasis of the skeletal system. Studies on the coupling mechanism in physiological and pathological settings have revealed that osteoclasts or osteoclastic bone resorption promote bone formation through the production of diverse coupling factors. The classical coupling factors are the molecules that promote bone formation after resorption, but there may be distinct mechanisms at work in various phases of bone remodeling. A recent study revealed that the Semaphorin 4D expressed by osteoclasts inhibits bone formation, which represents a mechanism by which coupling is dissociated. Furthermore, it has been demonstrated that osteoblastic expression of Semaphorin 3A exerts an osteoprotective effect by both suppressing bone resorption and increasing bone formation. Thus, recent advances have made it increasingly clear that bone remodeling is regulated by not only classical coupling factors, but also molecules that mediate cell-cell communication among bone cells. We propose that such factors be called bone cell communication factors, which control the delicate balance of the interaction of bone cells so as to maintain bone homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007;7:292–304. - PubMed
-
- Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 1965;206:489–490. - PubMed
-
- Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–2261. - PubMed
-
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005;11:76–81. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
