Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2
- PMID: 24171444
- PMCID: PMC3866584
- DOI: 10.1021/bi4010649
Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2
Abstract
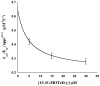
Lipoxygenases, important enzymes in inflammation, can regulate their substrate specificity by allosteric interactions with their own hydroperoxide products. In this work, addition of both 13-(S)-hydroxy-(9Z,11E)-octadecadienoic acid [13-(S)-HODE] and 13-(S)-hydroperoxy-(6Z,9Z,11E)-octadecatrienoic acid to human epithelial 15-lipoxygenase-2 (15-LOX-2) increases the kcat/KM substrate specificity ratio of arachidonic acid (AA) and γ-linolenic acid (GLA) by 4-fold. 13-(S)-HODE achieves this change by activating kcat/KM(AA) but inhibiting kcat/KM(GLA), which indicates that the allosteric structural changes at the active site discriminate between the length and unsaturation differences of AA and GLA to achieve opposite kinetic effects. The substrate specificity ratio is further increased, 11-fold in total, with an increase in pH, suggesting mechanistic differences between the pH and allosteric effects. Interestingly, the loss of the PLAT domain affects substrate specificity but does not eliminate the allosteric properties of 15-LOX-2, indicating that the allosteric site is located in the catalytic domain. However, the removal of the PLAT domain does change the magnitude of the allosteric effect. These data suggest that the PLAT domain moderates the communication pathway between the allosteric and catalytic sites, thus affecting substrate specificity. These results are discussed in the context of protein dimerization and other structural changes.
Figures







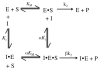
Similar articles
-
Structural and Functional Biology of Mammalian ALOX Isoforms with Particular Emphasis on Enzyme Dimerization and Their Allosteric Properties.Int J Mol Sci. 2024 Nov 9;25(22):12058. doi: 10.3390/ijms252212058. Int J Mol Sci. 2024. PMID: 39596127 Free PMC article. Review.
-
Kinetic and structural investigations of the allosteric site in human epithelial 15-lipoxygenase-2.Biochemistry. 2009 Sep 15;48(36):8721-30. doi: 10.1021/bi9009242. Biochemistry. 2009. PMID: 19645454 Free PMC article.
-
Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation.Biochemistry. 2008 Jul 15;47(28):7364-75. doi: 10.1021/bi800550n. Epub 2008 Jun 21. Biochemistry. 2008. PMID: 18570379 Free PMC article.
-
In Vitro Biosynthetic Pathway Investigations of Neuroprotectin D1 (NPD1) and Protectin DX (PDX) by Human 12-Lipoxygenase, 15-Lipoxygenase-1, and 15-Lipoxygenase-2.Biochemistry. 2021 Jun 8;60(22):1741-1754. doi: 10.1021/acs.biochem.0c00931. Epub 2021 May 24. Biochemistry. 2021. PMID: 34029049 Free PMC article.
-
Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications.Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:263-90. doi: 10.1016/s0090-6980(02)00035-7. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432923 Review.
Cited by
-
Fatty Acid Allosteric Regulation of C-H Activation in Plant and Animal Lipoxygenases.Molecules. 2020 Jul 24;25(15):3374. doi: 10.3390/molecules25153374. Molecules. 2020. PMID: 32722330 Free PMC article. Review.
-
The enzymology of human eicosanoid pathways: the lipoxygenase branches.Mol Biol Rep. 2020 Sep;47(9):7189-7207. doi: 10.1007/s11033-020-05698-8. Epub 2020 Aug 3. Mol Biol Rep. 2020. PMID: 32748021 Review.
-
The Arabidopsis PLAT domain protein1 is critically involved in abiotic stress tolerance.PLoS One. 2014 Nov 14;9(11):e112946. doi: 10.1371/journal.pone.0112946. eCollection 2014. PLoS One. 2014. PMID: 25396746 Free PMC article.
-
Stepwise processing analyses of the single-turnover PCSK9 protease reveal its substrate sequence specificity and link clinical genotype to lipid phenotype.J Biol Chem. 2018 Feb 9;293(6):1875-1886. doi: 10.1074/jbc.RA117.000754. Epub 2017 Dec 19. J Biol Chem. 2018. PMID: 29259136 Free PMC article.
-
Structural and Functional Biology of Mammalian ALOX Isoforms with Particular Emphasis on Enzyme Dimerization and Their Allosteric Properties.Int J Mol Sci. 2024 Nov 9;25(22):12058. doi: 10.3390/ijms252212058. Int J Mol Sci. 2024. PMID: 39596127 Free PMC article. Review.
References
-
- Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H, Walther M. Molecular enzymology of lipoxygenases. Archives of biochemistry and biophysics. 2010;503:161–174. - PubMed
-
- Brash AR. Lipoxygenases: Occurrence, Functions, Catalysis and Acquisition of Substrate. J Biol Chem. 1999;274:23679–23682. - PubMed
-
- Noguchi N, Yamashita H, Hamahara J, Nakamura A, Kuhn H, Niki E. The specificity of lipoxygenase-catalyzed lipid peroxidation and the effects of radical-scavenging antioxidants. Biol Chem. 2002;383:619–626. - PubMed
-
- Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

