Autologous transplantation as consolidation for aggressive non-Hodgkin's lymphoma
- PMID: 24171516
- PMCID: PMC3985418
- DOI: 10.1056/NEJMoa1301077
Autologous transplantation as consolidation for aggressive non-Hodgkin's lymphoma
Abstract
Background: The efficacy of autologous stem-cell transplantation during the first remission in patients with diffuse, aggressive non-Hodgkin's lymphoma classified as high-intermediate risk or high risk on the International Prognostic Index remains controversial and is untested in the rituximab era.
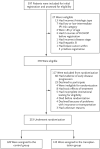
Methods: We treated 397 patients who had disease with an age-adjusted classification of high risk or high-intermediate risk with five cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP plus rituximab. Patients with a response were randomly assigned to receive three additional cycles of induction chemotherapy (control group) or one additional cycle of induction chemotherapy followed by autologous stem-cell transplantation (transplantation group). The primary efficacy end points were 2-year progression-free survival and overall survival.
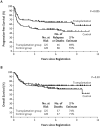
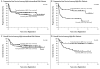
Results: Of 370 induction-eligible patients, 253 were randomly assigned to the transplantation group (125) or the control group (128). Forty-six patients in the transplantation group and 68 in the control group had disease progression or died, with 2-year progression-free survival rates of 69 and 55%, respectively (hazard ratio in the control group vs. the transplantation group, 1.72; 95% confidence interval [CI], 1.18 to 2.51; P=0.005). Thirty-seven patients in the transplantation group and 47 in the control group died, with 2-year overall survival rates of 74 and 71%, respectively (hazard ratio, 1.26; 95% CI, 0.82 to 1.94; P=0.30). Exploratory analyses showed a differential treatment effect according to risk level for both progression-free survival (P=0.04 for interaction) and overall survival (P=0.01 for interaction). Among high-risk patients, the 2-year overall survival rate was 82% in the transplantation group and 64% in the control group.
Conclusions: Early autologous stem-cell transplantation improved progression-free survival among patients with high-intermediate-risk or high-risk disease who had a response to induction therapy. Overall survival after transplantation was not improved, probably because of the effectiveness of salvage transplantation. (Funded by the National Cancer Institute, Department of Health and Human Services, and others; SWOG-9704 ClinicalTrials.gov number, NCT00004031.).
Conflict of interest statement
Dr. Porcu reports receiving consulting fees from Hospira and Medicis, lecture fees from Physician Education Resources, honoraria from Easton Associates, ClearView Health Care Partners, and E Squared Communications, and grant support through his institution from Millennium. Dr. Kahl reports receiving payment for board membership from Roche, Seattle Genetics, Millennium, and Cell Therapeutics and consulting fees from Genentech and Celgene. Dr. Miller reports receiving grant support through his institution from Spectrum, Celgene, and Abbott. Dr. Tubbs reports receiving lecture fees from Ventana Medical Systems and grant support through his institution from Ventana Medical Systems and Abbott Molecular Vysis. Dr. Friedberg reports receiving consulting fees from Genentech, Lilly, and Trubion. Dr. LeBlanc reports holding a pending patent regarding the uses of diffuse large-B-cell lymphoma markers. Dr. Rimsza reports receiving lecture fees from Ventana Medical Systems, and grant support through her institution from Ventana Medical Systems, Spectrum Pharmaceuticals, and Merck. Dr. Fisher reports receiving consulting fees from Micromet, Bio Linx, Boehringer Ingelheim, Roche, and Pfizer. No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
Myeloablation for lymphoma--question answered?N Engl J Med. 2013 Oct 31;369(18):1750-1. doi: 10.1056/NEJMe1309182. N Engl J Med. 2013. PMID: 24171521 No abstract available.
-
The role of myeloablation for lymphoma.N Engl J Med. 2014 Feb 6;370(6):575-6. doi: 10.1056/NEJMc1314757. N Engl J Med. 2014. PMID: 24499218 No abstract available.
-
The role of myeloablation for lymphoma.N Engl J Med. 2014 Feb 6;370(6):574-5. doi: 10.1056/NEJMc1314757. N Engl J Med. 2014. PMID: 24499219 No abstract available.
References
-
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–5. - PubMed
-
- The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–94. - PubMed
-
- Haioun C, Lepage E, Gisselbrecht C, et al. Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: final analysis of the prospective LNH87-2 protocol — a Groupe d'Etude des Lymphomes de l'Adulte study. J Clin Oncol. 2000;18:3025–30. - PubMed
-
- Nademanee A, Molina A, O'Donnell MR, et al. Results of high-dose therapy and autologous bone marrow/stem cell transplantation during remission in poor-risk intermediated and high-grade lymphoma: International Index High and High-Intermediate Risk Group. Blood. 1997;90:3844–52. - PubMed
-
- Freedman AS, Takvorian T, Neuberg D, et al. Autologous bone marrow transplantation in poor-prognosis intermediate-grade and high-grade B-cell non-Hodgkin's lymphoma in first remission: a pilot study. J Clin Oncol. 1993;11:931–6. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- CA63845/CA/NCI NIH HHS/United States
- N01 CA046441/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA035431/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- U10 CA063844/CA/NCI NIH HHS/United States
- N01 CA004919/CA/NCI NIH HHS/United States
- CA21076/CA/NCI NIH HHS/United States
- CA37981/CA/NCI NIH HHS/United States
- U10 CA022433/CA/NCI NIH HHS/United States
- CA58416/CA/NCI NIH HHS/United States
- U10 CA027057/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- CA35261/CA/NCI NIH HHS/United States
- U10 CA037981/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- N01 CA035431/CA/NCI NIH HHS/United States
- CA22433/CA/NCI NIH HHS/United States
- CA047559/CA/NCI NIH HHS/United States
- U10 CA046113/CA/NCI NIH HHS/United States
- U10 CA045560/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- CA20319/CA/NCI NIH HHS/United States
- U10 CA063845/CA/NCI NIH HHS/United States
- U10 CA076447/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- N01 CA013612/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- U10 CA077202/CA/NCI NIH HHS/United States
- CA077202/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA013612/CA/NCI NIH HHS/United States
- U10 CA058416/CA/NCI NIH HHS/United States
- CA35281/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- CA58658/CA/NCI NIH HHS/United States
- CA077658/CA/NCI NIH HHS/United States
- U10 CA017145/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- CA45377/CA/NCI NIH HHS/United States
- U10 CA035281/CA/NCI NIH HHS/United States
- CA11083/CA/NCI NIH HHS/United States
- CA58861/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- CA76132/CA/NCI NIH HHS/United States
- N01 CA063844/CA/NCI NIH HHS/United States
- U10 CA035178/CA/NCI NIH HHS/United States
- U10 CA035261/CA/NCI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- CA76447/CA/NCI NIH HHS/United States
- CA17145/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- N01 CA035178/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- U10 CA058686/CA/NCI NIH HHS/United States
- U10 CA046441/CA/NCI NIH HHS/United States
- U10 CA045377/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- U10 CA020319/CA/NCI NIH HHS/United States
- CA46113/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
- CA58686/CA/NCI NIH HHS/United States
- CA76448/CA/NCI NIH HHS/United States
- U10 CA011083/CA/NCI NIH HHS/United States
- CA52654/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA052654/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U10 CA058861/CA/NCI NIH HHS/United States
- N01 CA045560/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
