The role of CCL5 in the ability of adipose tissue-derived mesenchymal stem cells to support repair of ischemic regions
- PMID: 24171667
- PMCID: PMC3928761
- DOI: 10.1089/scd.2013.0307
The role of CCL5 in the ability of adipose tissue-derived mesenchymal stem cells to support repair of ischemic regions
Abstract
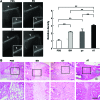
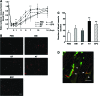
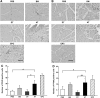
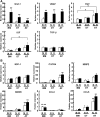
Mesenchymal stem cells (MSC) are multipotent and possess high proliferative activity, and thus are thought to be a reliable cell source for cell therapies. Here, we isolated MSC from adult tissues--bone marrow (BM-MSC), dental tissue (DT-MSC), and adipose tissue (AT-MSC)--to compare how autotransplantation of these MSC effectively supports the repair of bone fracture and ischemic tissue. An analysis by in vitro differentiation assays showed no significant difference among these MSC. The degree of calcification at the joint region of bone fracture was higher in mice transplanted with AT-MSC than in mice transplanted with BM-MSC or DT-MSC. To compare the abilities of MSC, characterize how those MSC affect the repair of ischemic tissue, vascular occlusion was performed by ligation of the femoral artery and vein. Of note, the blood flow in the ischemic region rapidly increased in mice injected with AT-MSC, as contrasted with mice injected with BM- or DT-MSC. The number of CD45- and F4/80-positive cells at the femoral region was higher in AT-MSC recipients than in recipients of BM-MSC or DT-MSC. We evaluated the mRNA expression of angiogenic and migration factors in MSC and found the expression of CCL5 mRNA was higher in AT-MSC than in BM-MSC or DT-MSC. Transplantation of AT-MSC with impaired expression of CCL5 clearly showed a significant delay in the recovery of blood flow compared with the control. These findings have fundamental implications for the modulation of AT-MSC in the repair of vasculature and bone fracture.
Figures


References
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49 - PubMed
-
- Herzog EL, Chai L. and Krause DS. (2003). Plasticity of marrow-derived stem cells. Blood 102:3483–3493 - PubMed
-
- Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, et al. (2002). Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 360:427–435 - PubMed
-
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC. and Phinney DG. (2003). Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89:1235–1249 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

