Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion
- PMID: 24172073
- PMCID: PMC3928683
- DOI: 10.1089/scd.2013.0274
Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion
Abstract
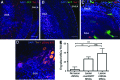
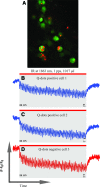
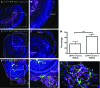
A loss of sensory hair cells or spiral ganglion neurons from the inner ear causes deafness, affecting millions of people. Currently, there is no effective therapy to repair the inner ear sensory structures in humans. Cochlear implantation can restore input, but only if auditory neurons remain intact. Efforts to develop stem cell-based treatments for deafness have demonstrated progress, most notably utilizing embryonic-derived cells. In an effort to bypass limitations of embryonic or induced pluripotent stem cells that may impede the translation to clinical applications, we sought to utilize an alternative cell source. Here, we show that adult human mesenchymal-like stem cells (MSCs) obtained from nasal tissue can repair spiral ganglion loss in experimentally lesioned cochlear cultures from neonatal rats. Stem cells engraft into gentamicin-lesioned organotypic cultures and orchestrate the restoration of the spiral ganglion neuronal population, involving both direct neuronal differentiation and secondary effects on endogenous cells. As a physiologic assay, nasal MSC-derived cells engrafted into lesioned spiral ganglia demonstrate responses to infrared laser stimulus that are consistent with those typical of excitable cells. The addition of a pharmacologic activator of the canonical Wnt/β-catenin pathway concurrent with stem cell treatment promoted robust neuronal differentiation. The availability of an effective adult autologous cell source for inner ear tissue repair should contribute to efforts to translate cell-based strategies to the clinic.
Figures




References
-
- Pittenger MF. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 - PubMed
-
- Murrell W, Feron F, Wetzig A, Cameron N, Splatt K, Bellette B, Bianco J, Perry C, Lee G. and Mackay-Sim A. (2005). Multipotent stem cells from adult olfactory mucosa. Dev Dyn 233:496–515 - PubMed
-
- Jakob M, Hemeda H, Janeschik S, Bootz F, Rotter N, Lang S. and Brandau S. (2010). Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells. Stem Cells Dev 19:635–644 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

