Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory
- PMID: 24172155
- PMCID: PMC3911413
- DOI: 10.1128/JCM.02378-13
Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory
Abstract
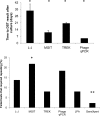
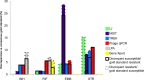
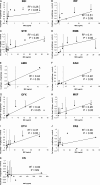
Given the increases in drug-resistant tuberculosis, laboratory capacities for drug susceptibility testing are being scaled up worldwide. A laboratory must decide among several endorsed methodologies. We evaluated 87 Mycobacterium tuberculosis isolates for concordance of susceptibility results across six methods: the L-J proportion method, MGIT 960 SIRE AST, Gene/Xpert MTB/RIF, GenoType MTBDRplus line probe assay, MycoTB MIC plate, and a laboratory-developed mycobacteriophage quantitative PCR (qPCR)-based method. Most (80%) isolates were multidrug resistant. Of the culture-based methods, the mycobacteriophage qPCR method was fastest, the L-J proportion method was the slowest, and the MGIT method required the most repeat testing (P < 0.05). For isoniazid (INH), 82% of isolates were susceptible by all methods or resistant by all methods, whereas for rifampin (RIF), ethambutol (EMB), and streptomycin (STR), such complete concordance was observed in 77%, 50%, and 51% of isolates, respectively (P < 0.05 for INH or RIF versus EMB or STR). The discrepancies of EMB and STR stemmed largely from diminished concordance of the MGIT EMB results (kappa coefficient range, 0.26 to 0.30) and the L-J STR result (kappa range, 0.35 to 0.45) versus other methods. Phage qPCR and the MycoTB MIC plate were the only methods that yielded second-line susceptibilities and revealed significant quantitative correlations for all drugs except cycloserine, as well as moderate to excellent kappa coefficients for all drugs except for para-aminosalicylic acid. In summary, the performance of M. tuberculosis susceptibility testing differs by platform and by drug. Laboratories should carefully consider these factors before choosing one methodology, particularly in settings where EMB and STR results are clinically important.
Figures

References
-
- WHO 1998. Guidelines for surveillance of drug resistance in tuberculosis. WHO and the International Union Against Tuberculosis and Lung Disease. Int. J. Tuberc. Lung Dis. 2:72–89 - PubMed
-
- WHO 2008. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB): policy statement. WHO, Geneva, Switzerland
-
- WHO 2007. Use of liquid TB culture and drug susceptibility testing (DST) in low and medium income settings. Summary report of the Expert Group Meeting on the Use of Liquid Culture Media. WHO, Geneva, Switzerland
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

