Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae)
- PMID: 24172213
- PMCID: PMC3853722
- DOI: 10.3390/md11114176
Anti HSV-1 activity of halistanol sulfate and halistanol sulfate C isolated from Brazilian marine sponge Petromica citrina (Demospongiae)
Abstract
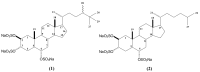
The n-butanol fraction (BF) obtained from the crude extract of the marine sponge Petromica citrina, the halistanol-enriched fraction (TSH fraction), and the isolated compounds halistanol sulfate (1) and halistanol sulfate C (2), were evaluated for their inhibitory effects on the replication of the Herpes Simplex Virus type 1 (HSV-1, KOS strain) by the viral plaque number reduction assay. The TSH fraction was the most effective against HSV-1 replication (SI = 15.33), whereas compounds 1 (SI = 2.46) and 2 (SI = 1.95) were less active. The most active fraction and these compounds were also assayed to determine the viral multiplication step(s) upon which they act as well as their potential synergistic effects. The anti-HSV-1 activity detected was mediated by the inhibition of virus attachment and by the penetration into Vero cells, the virucidal effect on virus particles, and by the impairment in levels of ICP27 and gD proteins of HSV-1. In summary, these results suggest that the anti-HSV-1 activity of TSH fraction detected is possibly related to the synergic effects of compounds 1 and 2.
Figures



Similar articles
-
TSH fraction from Petromica citrina: A potential marine natural product for the treatment of sepsis by Methicillin-resistant Staphylococcus aureus (MRSA).Biomed Pharmacother. 2018 Dec;108:1759-1766. doi: 10.1016/j.biopha.2018.10.023. Epub 2018 Oct 12. Biomed Pharmacother. 2018. PMID: 30372879
-
Antibacterial activity and cytotoxicity analysis of halistanol trisulphate from marine sponge Petromica citrina.J Antimicrob Chemother. 2012 Oct;67(10):2396-400. doi: 10.1093/jac/dks229. Epub 2012 Jun 22. J Antimicrob Chemother. 2012. PMID: 22729926
-
Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotus peltatus: an ethnomedicine from Andaman Islands.Virol J. 2012 May 24;9:98. doi: 10.1186/1743-422X-9-98. Virol J. 2012. PMID: 22624581 Free PMC article.
-
Inhibitory effect of Spirogyra spp. algal extracts against herpes simplex virus type 1 and 2 infection.J Appl Microbiol. 2018 Jun;124(6):1441-1453. doi: 10.1111/jam.13729. Epub 2018 Mar 13. J Appl Microbiol. 2018. PMID: 29532624
-
Novel HIV-inhibitory halistanol sulfates F-H from a marine sponge, Pseudoaxinissa digitata.J Nat Prod. 1994 Jan;57(1):164-7. doi: 10.1021/np50103a026. J Nat Prod. 1994. PMID: 8158159
Cited by
-
Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses.Viruses. 2021 Jul 16;13(7):1386. doi: 10.3390/v13071386. Viruses. 2021. PMID: 34372592 Free PMC article. Review.
-
Marine Demospongiae: A Challenging Treasure of Bioactive Compounds.Mar Drugs. 2022 Mar 31;20(4):244. doi: 10.3390/md20040244. Mar Drugs. 2022. PMID: 35447918 Free PMC article. Review.
-
Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development.Viruses. 2020 Jan 29;12(2):154. doi: 10.3390/v12020154. Viruses. 2020. PMID: 32013134 Free PMC article. Review.
-
New Trisulfated Steroids from the Vietnamese Marine Sponge Halichondria vansoesti and Their PSA Expression and Glucose Uptake Inhibitory Activities.Mar Drugs. 2019 Jul 27;17(8):445. doi: 10.3390/md17080445. Mar Drugs. 2019. PMID: 31357591 Free PMC article.
-
Antiproliferative and antioxidant properties of nematocysts crude venom from jellyfish Acromitus flagellatus against human cancer cell lines.Saudi J Biol Sci. 2021 Mar;28(3):1954-1961. doi: 10.1016/j.sjbs.2020.12.047. Epub 2021 Jan 2. Saudi J Biol Sci. 2021. PMID: 33732081 Free PMC article.
References
-
- Nakakawa E., Reynold S.J. The management of herpes simplex virus infections in HIV infected patients: Current issues and the role of cidofovir. Virus Adapt. Treat. 2011;3:35–43.
-
- Mayer A.M.S., Rodríguez A.D., Berlinck R.G.S., Fusetani N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011;153:191–222. doi: 10.1016/j.cbpc.2010.08.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

