Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice
- PMID: 24172214
- PMCID: PMC3853723
- DOI: 10.3390/md11114193
Anti-diabetic effect of balanced deep-sea water and its mode of action in high-fat diet induced diabetic mice
Abstract
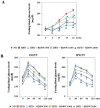
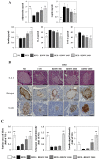
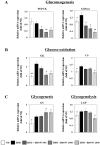
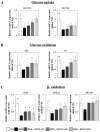
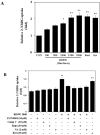
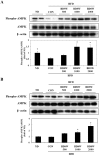
In this study, we investigated the effects of balanced deep-sea water (BDSW) on hyperglycemia and glucose intolerance in high-fat diet (HFD)-induced diabetic C57BL/6J mice. BDSW was prepared by mixing deep-sea water (DSW) mineral extracts and desalinated water to give a final hardness of 500-2000. Mice given an HFD with BDSW showed lowered fasting plasma glucose levels compared to HFD-fed mice. Oral and intraperitoneal glucose tolerance tests showed that BDSW improves impaired glucose tolerance in HFD-fed mice. Histopathological evaluation of the pancreas showed that BDSW recovers the size of the pancreatic islets of Langerhans, and increases the secretion of insulin and glucagon in HFD-fed mice. Quantitative reverse transcription polymerase chain reaction results revealed that the expression of hepatic genes involved in glucogenesis, glycogenolysis and glucose oxidation were suppressed, while those in glucose uptake, β-oxidation, and glucose oxidation in muscle were increased in mice fed HFD with BDSW. BDSW increased AMP-dependent kinase (AMPK) phosphorylation in 3T3-L1 pre- and mature adipocytes and improved impaired AMPK phosphorylation in the muscles and livers of HFD-induced diabetic mice. BDSW stimulated phosphoinositol-3-kinase and AMPK pathway-mediated glucose uptake in 3T3-L1 adipocytes. Taken together, these results suggest that BDSW has potential as an anti-diabetic agent, given its ability to suppress hyperglycemia and improve glucose intolerance by increasing glucose uptake.
Figures

Similar articles
-
Stimulatory Effect of Balanced Deep-Sea Water Containing Chitosan Oligosaccharides on Glucose Uptake in C2C12 Myotubes.Mar Biotechnol (NY). 2016 Aug;18(4):475-84. doi: 10.1007/s10126-016-9709-5. Epub 2016 May 23. Mar Biotechnol (NY). 2016. PMID: 27215753
-
Modulation of glucose metabolism by balanced deep-sea water ameliorates hyperglycemia and pancreatic function in streptozotocin-induced diabetic mice.PLoS One. 2014 Jul 11;9(7):e102095. doi: 10.1371/journal.pone.0102095. eCollection 2014. PLoS One. 2014. PMID: 25013896 Free PMC article.
-
The inhibiting effect of the Coptis chinensis polysaccharide on the type II diabetic mice.Biomed Pharmacother. 2016 Jul;81:111-119. doi: 10.1016/j.biopha.2016.03.038. Epub 2016 Apr 12. Biomed Pharmacother. 2016. PMID: 27261584
-
Effects of balanced deep-sea water on adipocyte hypertrophy and liver steatosis in high-fat, diet-induced obese mice.Obesity (Silver Spring). 2014 Jul;22(7):1669-78. doi: 10.1002/oby.20740. Epub 2014 Mar 25. Obesity (Silver Spring). 2014. PMID: 24634394
-
Magnesium and calcium-enriched deep-sea water promotes mitochondrial biogenesis by AMPK-activated signals pathway in 3T3-L1 preadipocytes.Biomed Pharmacother. 2016 Oct;83:477-484. doi: 10.1016/j.biopha.2016.07.009. Epub 2016 Jul 18. Biomed Pharmacother. 2016. PMID: 27434863
Cited by
-
Stimulatory Effect of Balanced Deep-Sea Water Containing Chitosan Oligosaccharides on Glucose Uptake in C2C12 Myotubes.Mar Biotechnol (NY). 2016 Aug;18(4):475-84. doi: 10.1007/s10126-016-9709-5. Epub 2016 May 23. Mar Biotechnol (NY). 2016. PMID: 27215753
-
Dietary Consumption on Glycemic Control Among Prediabetes: A Review of the Literature.SAGE Open Nurs. 2023 Dec 20;9:23779608231218189. doi: 10.1177/23779608231218189. eCollection 2023 Jan-Dec. SAGE Open Nurs. 2023. PMID: 38130469 Free PMC article. Review.
-
Mineral-rich Jeju lava sea water suppresses lipid accumulation in 3T3-L1 adipocytes and ameliorates high-fat diet-induced obesity in C57BL/6 J mice.Food Sci Biotechnol. 2021 Feb 6;30(2):299-304. doi: 10.1007/s10068-020-00859-8. eCollection 2021 Feb. Food Sci Biotechnol. 2021. PMID: 33732520 Free PMC article.
-
Deep Sea Water Inhibited Pancreatic β-Cell Apoptosis and Regulated Glucose Homeostasis by Affecting Lipid Metabolism in Db/Db Mice.Diabetes Metab Syndr Obes. 2023 Jan 27;16:245-258. doi: 10.2147/DMSO.S395053. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 36760598 Free PMC article.
-
Mineral-Enriched Deep-Sea Water Modulates Lactate Metabolism via PGC-1α-Mediated Metabolic Reprogramming.Mar Drugs. 2019 Oct 27;17(11):611. doi: 10.3390/md17110611. Mar Drugs. 2019. PMID: 31717879 Free PMC article.
References
-
- Tahrani A.A., Piya M.K., Kennedy A., Barnett A.H. Glycaemic control in type 2 diabetes: Targets and new therapies. Pharmacol. Ther. 2010;125:328–361. - PubMed
-
- Lee T.I., Chen Y.C., Kao Y.H., Hsiao F.C., Lin Y.K., Chen Y.J. Rosiglitazone induces arrhythmogenesis in diabetic hypertensive rats with calcium handling alteration. Int. J. Cardiol. 2011;165:299–307. - PubMed
-
- Moller D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. - PubMed
-
- Liu Q., Chen L., Hu L., Guo Y., Shen X. Small molecules from natural sources, targeting signaling pathways in diabetes. Biochim. Biophys. Acta. 2010;1799:854–865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

