Short-term exposure to oleandrin enhances responses to IL-8 by increasing cell surface IL-8 receptors
- PMID: 24172227
- PMCID: PMC4105924
- DOI: 10.1111/bph.12493
Short-term exposure to oleandrin enhances responses to IL-8 by increasing cell surface IL-8 receptors
Abstract
Background and purpose: One of the first steps in host defence is the migration of leukocytes. IL-8 and its receptors are a chemokine system essential to such migration. Up-regulation of these receptors would be a viable strategy to treat dysfunctional host defence. Here, we studied the effects of the plant glycoside oleandrin on responses to IL-8 in a human monocytic cell line.
Experimental approach: U937 cells were incubated with oleandrin (1-200 ng mL(-1) ) for either 1 h (pulse) or for 24 h (non-pulse). Apoptosis; activation of NF-κB, AP-1 and NFAT; calcineurin activity and IL-8 receptors (CXCR1 and CXCR2) were measured using Western blotting, RT-PCR and reporter gene assays.
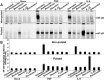
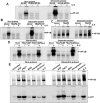
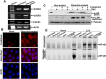
Key results: Pulse exposure to oleandrin did not induce apoptosis or cytoxicity as observed after non-pulse exposure. Pulse exposure enhanced activation of NF-κB induced by IL-8 but not that induced by TNF-α, IL-1, EGF or LPS. Exposure to other apoptosis-inducing compounds (azadirachtin, resveratrol, thiadiazolidine, or benzofuran) did not enhance activation of NF-κB. Pulse exposure to oleandrin increased expression of IL-8 receptors and chemotaxis, release of enzymes and activation of NF-κB, NFAT and AP-1 along with increased IL-8-mediated calcineurin activation, and wound healing. Pulse exposure increased numbers of cell surface IL-8 receptors.
Conclusions and implications: Short-term (1 h; pulse) exposure to a toxic glycoside oleandrin, enhanced biological responses to IL-8 in monocytic cells, without cytoxicity. Pulse exposure to oleandrin could provide a viable therapy for those conditions where leukocyte migration is defective.
Keywords: IL-8 receptor; NF-κB; apoptosis; cardiac glycoside; chemotaxis.
© 2013 The British Pharmacological Society.
Figures





References
-
- Abbasi S, Su B, Kellems RE, Yang J, Xia Y. The essential role of MEKK3 signaling in angiotensin II-induced calcineurin/nuclear factor of activated T-cells activation. J Biol Chem. 2005;280:36737–36746. - PubMed
-
- Bidyasar S, Kurzrock R, Falchook GS, Naing A, Wheler JJ, Durand J, et al. A first-in-human phase I trial of PBI-05204 (oleandrin), an inhibitor of Akt, FGF-2, NF-kB, and p70S6K in advanced solid tumor patients. J Clin Oncol. 2009;27:15S. Abst 3537.
-
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

