MicroRNAs and other non-coding RNAs as targets for anticancer drug development
- PMID: 24172333
- PMCID: PMC4548803
- DOI: 10.1038/nrd4140
MicroRNAs and other non-coding RNAs as targets for anticancer drug development
Abstract
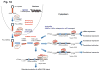
The first cancer-targeted microRNA (miRNA) drug - MRX34, a liposome-based miR-34 mimic - entered Phase I clinical trials in patients with advanced hepatocellular carcinoma in April 2013, and miRNA therapeutics are attracting special attention from both academia and biotechnology companies. Although miRNAs are the most studied non-coding RNAs (ncRNAs) to date, the importance of long non-coding RNAs (lncRNAs) is increasingly being recognized. Here, we summarize the roles of miRNAs and lncRNAs in cancer, with a focus on the recently identified novel mechanisms of action, and discuss the current strategies in designing ncRNA-targeting therapeutics, as well as the associated challenges.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

