Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys
- PMID: 24172905
- PMCID: PMC4017780
- DOI: 10.1038/nature12744
Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys
Abstract
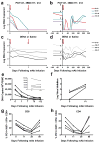
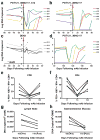
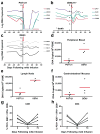
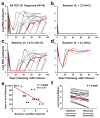
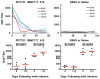
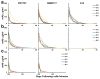
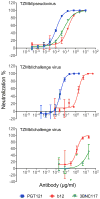
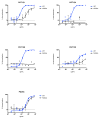
Human immunodeficiency virus type 1 (HIV-1)-specific monoclonal antibodies with extraordinary potency and breadth have recently been described. In humanized mice, combinations of monoclonal antibodies have been shown to suppress viraemia, but the therapeutic potential of these monoclonal antibodies has not yet been evaluated in primates with an intact immune system. Here we show that administration of a cocktail of HIV-1-specific monoclonal antibodies, as well as the single glycan-dependent monoclonal antibody PGT121, resulted in a rapid and precipitous decline of plasma viraemia to undetectable levels in rhesus monkeys chronically infected with the pathogenic simian-human immunodeficiency virus SHIV-SF162P3. A single monoclonal antibody infusion afforded up to a 3.1 log decline of plasma viral RNA in 7 days and also reduced proviral DNA in peripheral blood, gastrointestinal mucosa and lymph nodes without the development of viral resistance. Moreover, after monoclonal antibody administration, host Gag-specific T-lymphocyte responses showed improved functionality. Virus rebounded in most animals after a median of 56 days when serum monoclonal antibody titres had declined to undetectable levels, although, notably, a subset of animals maintained long-term virological control in the absence of further monoclonal antibody infusions. These data demonstrate a profound therapeutic effect of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys as well as an impact on host immune responses. Our findings strongly encourage the investigation of monoclonal antibody therapy for HIV-1 in humans.
Conflict of interest statement
The authors declare no competing financial interests. M.C.N. and D.R.B. are co-inventors on patents covering the mAbs utilized in the present study.
Figures
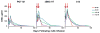
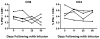

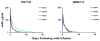
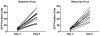
Comment in
-
HIV: Antibodies advance the search for a cure.Nature. 2013 Nov 14;503(7475):207-8. doi: 10.1038/nature12703. Epub 2013 Oct 30. Nature. 2013. PMID: 24172894 No abstract available.
-
HIV: advancing the antibody approach.Nat Rev Immunol. 2013 Dec;13(12):846-7. doi: 10.1038/nri3573. Nat Rev Immunol. 2013. PMID: 24409525 No abstract available.
References
-
- Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007387/AI/NIAID NIH HHS/United States
- U19 AI078526/AI/NIAID NIH HHS/United States
- AI10063/AI/NIAID NIH HHS/United States
- P01 AI100148/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI084794/AI/NIAID NIH HHS/United States
- AI078526/AI/NIAID NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- U19 AI096040/AI/NIAID NIH HHS/United States
- AI100663/AI/NIAID NIH HHS/United States
- R37 AI055332/AI/NIAID NIH HHS/United States
- AI055332/AI/NIAID NIH HHS/United States
- AI100148/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R56 AI091514/AI/NIAID NIH HHS/United States
- UM1 AI100663/AI/NIAID NIH HHS/United States
- AI095985/AI/NIAID NIH HHS/United States
- R01 AI055332/AI/NIAID NIH HHS/United States
- AI096040/AI/NIAID NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- AI084794/AI/NIAID NIH HHS/United States
- U19 AI095985/AI/NIAID NIH HHS/United States
- P40 OD012217/OD/NIH HHS/United States
- U19 AI066305/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

