Immunosuppression facilitates the reactivation of latent papillomavirus infections
- PMID: 24173230
- PMCID: PMC3911712
- DOI: 10.1128/JVI.02589-13
Immunosuppression facilitates the reactivation of latent papillomavirus infections
Abstract
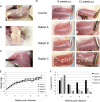
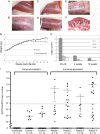
At mucosal sites, papillomavirus genomes can persist in the epithelial basal layer following immune-mediated regression. Subsequent T-cell depletion stimulates a 3- to 5-log increase in the viral copy number, to levels associated with productive infection. Reappearance of microlesions was rare within the short time frame of our experiments but was observed in one instance. Our studies provide direct evidence that immunosuppression can trigger the reactivation of latent papillomavirus genomes, as previously proposed in humans.
Figures


References
-
- Jamieson DJ, Duerr A, Burk R, Klein RS, Paramsothy P, Schuman P, Cu-Uvin S, Shah K. 2002. Characterization of genital human papillomavirus infection in women who have or who are at risk of having HIV infection. Am. J. Obstet. Gynecol. 186:21–27 - PubMed
-
- Moscicki AB, Ellenberg JH, Vermund SH, Holland CA, Darragh T, Crowley-Nowick PA, Levin L, Wilson CM. 2000. Prevalence of and risks for cervical human papillomavirus infection and squamous intraepithelial lesions in adolescent girls: impact of infection with human immunodeficiency virus. Arch. Pediatr. Adolesc. Med. 154:127–134 - PubMed
-
- Ozsaran AA, Ates T, Dikmen Y, Zeytinoglu A, Terek C, Erhan Y, Ozacar T, Bilgic A. 1999. Evaluation of the risk of cervical intraepithelial neoplasia and human papilloma virus infection in renal transplant patients receiving immunosuppressive therapy. Eur. J. Gynaecol. Oncol. 20:127–130 - PubMed
-
- Palefsky JM, Minkoff H, Kalish LA, Levine A, Sacks HS, Garcia P, Young M, Melnick S, Miotti P, Burk R. 1999. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J. Natl. Cancer Inst. 91:226–236 - PubMed
-
- Paternoster DM, Cester M, Resente C, Pascoli I, Nanhorngue K, Marchini F, Boccagni P, Cillo U, Ribaldone R, Amoruso E, Cocca N, Cuccolo V, Bertolino M, Surico N, Stratta P. 2008. Human papilloma virus infection and cervical intraepithelial neoplasia in transplanted patients. Transplant. Proc. 40:1877–1880 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

