Synthesis and evaluation of 18F-labeled benzylguanidine analogs for targeting the human norepinephrine transporter
- PMID: 24173571
- PMCID: PMC3947152
- DOI: 10.1007/s00259-013-2558-9
Synthesis and evaluation of 18F-labeled benzylguanidine analogs for targeting the human norepinephrine transporter
Abstract
Purpose: Both (131)I- and (123)I-labeled meta-iodobenzylguanidine (MIBG) have been widely used in the clinic for targeted imaging of the norepinephrine transporter (NET). The human NET (hNET) gene has been imaged successfully with (124)I-MIBG positron emission tomography (PET) at time points of >24 h post-injection (p.i.). (18)F-labeled MIBG analogs may be ideal to image hNET expression at time points of <8 h p.i. We developed improved methods for the synthesis of known MIBG analogs, [(18)F]MFBG and [(18)F]PFBG and evaluated them in hNET reporter gene-transduced C6 rat glioma cells and xenografts.
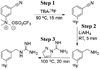
Methods: [(18)F]MFBG and [(18)F]PFBG were synthesized manually using a three-step synthetic scheme. Wild-type and hNET reporter gene-transduced C6 rat glioma cells and xenografts were used to comparatively evaluate the (18)F-labeled analogs with [(123)I]/[(124)I]MIBG.
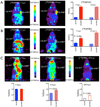
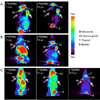
Results: The fluorination efficacy on benzonitrile was predominantly determined by the position of the trimethylammonium group. The para-isomer afforded higher yields (75 ± 7%) than meta-isomer (21 ± 5%). The reaction of [(18)F]fluorobenzylamine with 1H-pyrazole-1-carboximidamide was more efficient than with 2-methyl-2-thiopseudourea. The overall radiochemical yields (decay-corrected) were 11 ± 2% (n = 12) for [(18)F]MFBG and 41 ± 12% (n = 5) for [(18)F]PFBG, respectively. The specific uptakes of [(18)F]MFBG and [(18)F]PFBG were similar in C6-hNET cells, but 4-fold less than that of [(123)I]/[(124)I]MIBG. However, in vivo [(18)F]MFBG accumulation in C6-hNET tumors was 1.6-fold higher than that of [(18)F]PFBG at 1 h p.i., whereas their uptakes were similar at 4 h. Despite [(18)F]MFBG having a 2.8-fold lower affinity to hNET and approximately 4-fold lower cell uptake in vitro compared to [(123)I]/[(124)I]MIBG, PET imaging demonstrated that [(18)F]MFBG was able to visualize C6-hNET xenografts better than [(124)I]MIBG. Biodistribution studies showed [(18)F]MFBG and (123)I-MIBG had a similar tumor accumulation, which was lower than that of no-carrier-added [(124)I]MIBG, but [(18)F]MFBG showed a significantly more rapid body clearance and lower uptake in most non-targeting organs.
Conclusion: [(18)F]MFBG and [(18)F]PFBG were synthesized in reasonable radiochemical yields under milder conditions. [(18)F]MFBG is a better PET ligand to image hNET expression in vivo at 1-4 h p.i. than both [(18)F]PFBG and [(123)I]/[(124)I]MIBG.
Conflict of interest statement
Figures

References
-
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. - PubMed
-
- Axelrod J, Kopin IJ. The uptake, storage, release and metabolism of noradrenaline in sympathetic nerves. Prog Brain Res. 1969;31:21–32. - PubMed
-
- Anton M, Wagner B, Haubner R, Bodenstein C, Essien BE, Bonisch H, et al. Use of the norepinephrine transporter as a reporter gene for non-invasive imaging of genetically modified cells. J Gene Med. 2004;6:119–126. - PubMed
-
- Moroz MA, Serganova I, Zanzonico P, Ageyeva L, Beresten T, Dyomina E, et al. Imaging hNET reporter gene expression with 124I-MIBG. J Nucl Med. 2007;48:827–836. - PubMed
-
- Grunwald F, Ezziddin S. 131I–metaiodobenzylguanidine therapy of neuroblastoma and other neuroendocrine tumors. Semin Nucl Med. 2010;40:153–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

