A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression
- PMID: 24173623
- PMCID: PMC3945214
- DOI: 10.1007/s00213-013-3316-1
A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression
Abstract
Rationale: Postpartum depression (PMD) occurs in roughly 10 % of postpartum women and negatively impacts the mother and her offspring, but there are few placebo-controlled studies of antidepressant treatment in this population.
Objective: The objective was this study is to compare the selective serotonin reuptake inhibitor (SSRI) sertraline to placebo for treating PMD.
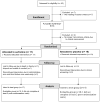
Methods: This was a single-center, 6-week, randomized double-blind placebo-controlled trial of sertraline with a 1-week placebo lead-in. The participants (n = 38) were women with depression onset within 3 months of delivery; a subset (n = 27) met strict DSM-IV criteria for PMD (onset within 4 weeks of delivery). The participants were prescribed sertraline 50 mg or placebo daily to a maximum of 200 mg/day. Primary outcome variables were the Hamilton Depression Rating Scale (HAM-D) and Clinical Global Impressions (CGI) scores, which were used to determine the rates of response and remission.
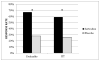
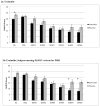
Results: Sertraline produced a significantly greater response rate (59 %) than placebo (26 %) and a more than twofold increased remission rate (53 % vs. 21 %). Mixed models did not reveal significant group by time effects, although in the subset of women who met the DSM-IV criteria, there was a statistically significant group by time effect for the HAM-D, Hamilton Anxiety Rating Scale (HAM-A), and CGI.
Conclusions: Women with PMD are more likely to have a remission of their depression with sertraline treatment, a finding that is more pronounced in women who have onset of depression within 4 weeks of childbirth. These data support the continued use of 4 weeks for the DSM-5 postpartum onset specifier for major depressive disorder.
Conflict of interest statement
Conflicts of Interest: Dr. Epperson has received research grant support from Pfizer, Eli Lilly, Shire, and Novartis, and honoraria from Pfizer, Eli Lilly, and Glaxo Smith Kline. Dr. Epperson or a family member holds stock in Johnson and Johnson, Pfizer, Merck and Company, Abbvie, and Abbott. Dr. Price has received research support from Medtronic, Neuronetics, NIH, HRSA, and Neosync; he has served on advisory panels for Abbott and AstraZeneca; and he has served as a consultant to Gerson Lehrman, Wiley, Springer, Qatar National Research Fund, and Abbott. All other authors declare that they have no conflicts of interest.
Figures
References
-
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: 2000. text rev.
-
- Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166:191–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

