Substrate stiffness regulates solubility of cellular vimentin
- PMID: 24173714
- PMCID: PMC3873896
- DOI: 10.1091/mbc.E13-06-0326
Substrate stiffness regulates solubility of cellular vimentin
Abstract
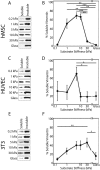
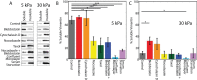
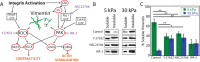
The intermediate filament protein vimentin is involved in the regulation of cell behavior, morphology, and mechanical properties. Previous studies using cells cultured on glass or plastic substrates showed that vimentin is largely insoluble. Although substrate stiffness was shown to alter many aspects of cell behavior, changes in vimentin organization were not reported. Our results show for the first time that mesenchymal stem cells (hMSCs), endothelial cells, and fibroblasts cultured on different-stiffness substrates exhibit biphasic changes in vimentin detergent solubility, which increases from nearly 0 to 67% in hMSCs coincident with increases in cell spreading and membrane ruffling. When imaged, the detergent-soluble vimentin appears to consist of small fragments the length of one or several unit-length filaments. Vimentin detergent solubility decreases when these cells are subjected to serum starvation, allowed to form cell-cell contacts, after microtubule disruption, or inhibition of Rac1, Rho-activated kinase, or p21-activated kinase. Inhibiting myosin or actin assembly increases vimentin solubility on rigid substrates. These data suggest that in the mechanical environment in vivo, vimentin is more dynamic than previously reported and its assembly state is sensitive to stimuli that alter cellular tension and morphology.
Figures



References
-
- Ando S, Nakao K, Gohara R, Takasaki Y, Suehiro K, Oishi Y. Morphological analysis of glutaraldehyde-fixed vimentin intermediate filaments and assembly-intermediates by atomic force microscopy. Biochim Biophys Acta. 2004;1702:53–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

