Comparison of itraconazole, voriconazole, and posaconazole as oral antifungal prophylaxis in pediatric patients following allogeneic hematopoietic stem cell transplantation
- PMID: 24173819
- PMCID: PMC3953550
- DOI: 10.1007/s10096-013-1998-2
Comparison of itraconazole, voriconazole, and posaconazole as oral antifungal prophylaxis in pediatric patients following allogeneic hematopoietic stem cell transplantation
Abstract
Oral antifungal prophylaxis with extended-spectra azoles is widely used in pediatric patients after allogeneic hematopoietic stem cell transplantation (HSCT), while controlled studies for oral antifungal prophylaxis after bone marrow transplantation in children are not available. This survey analyzed patients who had received either itraconazole, voriconazole, or posaconazole. We focused on the safety, feasibility, and initial data of efficacy in a cohort of pediatric patients and adolescents after high-dose chemotherapy and HSCT. Fifty consecutive pediatric patients received itraconazole, 50 received voriconazole, and 50 pediatric patients received posaconazole after HSCT as oral antifungal prophylaxis. The observation period lasted from the start of oral prophylactic treatment with itraconazole, voriconazole, or posaconazole until two weeks after terminating the oral antifungal prophylaxis. No incidences of proven or probable invasive mycosis were observed during itraconazole, voriconazole, or posaconazole treatment. A total of five possible invasive fungal infections occurred, two in the itraconazole group (4%) and three in the voriconazole group (6%). The percentage of patients with adverse events potentially related to clinical drugs were 14% in the voriconazole group, 12% in the itraconazole group, and 8% in the posaconazole group. Itraconazole, voriconazole, and posaconazole showed comparable efficacy as antifungal prophylaxis in pediatric patients after allogeneic HSCT.
Figures
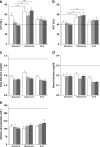
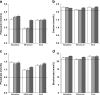
References
-
- Cuenca-Estrella M, Rodríguez-Tudela JL, Mellado E, Martínez-Suárez JV, Monzón A. Comparison of the in-vitro activity of voriconazole (UK-109,496), itraconazole and amphotericin B against clinical isolates of Aspergillus fumigatus. J Antimicrob Chemother. 1998;42:531–533. doi: 10.1093/jac/42.4.531. - DOI - PubMed
-
- Espinel-Ingroff A, Canton E, Gibbs D, Wang A. Correlation of Neo-Sensitabs tablet diffusion assay results on three different agar media with CLSI broth microdilution M27-A2 and disk diffusion M44-A results for testing susceptibilities of Candida spp. and Cryptococcus neoformans to amphotericin B, caspofungin, fluconazole, itraconazole, and voriconazole. J Clin Microbiol. 2007;45:858–864. doi: 10.1128/JCM.01900-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

