Risk of postprandial insulin resistance: the liver/vagus rapport
- PMID: 24174131
- PMCID: PMC4000159
- DOI: 10.1007/s11154-013-9281-5
Risk of postprandial insulin resistance: the liver/vagus rapport
Abstract
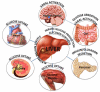
Ingestion of a meal is the greatest challenge faced by glucose homeostasis. The surge of nutrients has to be disposed quickly, as high concentrations in the bloodstream may have pathophysiological effects, and also properly, as misplaced reserves may induce problems in affected tissues. Thus, loss of the ability to adequately dispose of ingested nutrients can be expected to lead to glucose intolerance, and favor the development of pathologies. Achieving interplay of several organs is of upmost importance to maintain effectively postprandial glucose clearance, with the liver being responsible of orchestrating global glycemic control. This dogmatic role of the liver in postprandial insulin sensitivity is tightly associated with the vagus nerve. Herein, we uncover the behaviour of metabolic pathways determined by hepatic parasympathetic function status, in physiology and in pathophysiology. Likewise, the inquiry expands to address the impact of a modern lifestyle, especially one's feeding habits, on the hepatic parasympathetic nerve control of glucose metabolism.
Figures

References
-
- Garber AJ, Handelsman Y, Einhorn D, Bergman DA, Bloomgarden ZT, Fonseca V, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14(7):933–946. doi:8243877256X4103H [pii] - PubMed
-
- Zhang Y, Dall TM, Chen Y, Baldwin A, Yang W, Mann S, et al. Medical cost associated with prediabetes. Popul Health Manag. 2009;12(3):157–163. doi:10.1089/pop.2009.12302. - PubMed
-
- Gardete-Correia L, Boavida JM, Raposo JF, Mesquita AC, Fona C, Carvalho R, et al. First diabetes prevalence study in Portugal: PREVADIAB study. Diabet Med. 2010;27(8):879–881. doi:DME3017 [pii] 10.1111/j.1464-5491.2010.03017.x. - PubMed
-
- Ceriello A. Does postprandial blood glucose matter and why? Endocrinol Nutr. 2009;56S4:8–11. doi:S1575-0922(09)73508-9 [pii] 10.1016/S1575-0922(09)73508-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

