A randomized, double-blind, placebo-controlled, phase III study of the short-acting β1-adrenergic receptor blocker landiolol hydrochloride for coronary computed tomography angiography in Japanese patients with suspected ischemic cardiac disease
- PMID: 24174275
- PMCID: PMC3889825
- DOI: 10.1007/s40261-013-0149-y
A randomized, double-blind, placebo-controlled, phase III study of the short-acting β1-adrenergic receptor blocker landiolol hydrochloride for coronary computed tomography angiography in Japanese patients with suspected ischemic cardiac disease
Abstract
Objectives and background: The objective of this study was to investigate the image quality-improving and heart rate-lowering effects of landiolol hydrochloride (a short-acting β1-adrenergic receptor blocker) on coronary computed tomography angiography (CCTA). During CCTA, β-adrenergic receptor blockers have been commonly used to lower heart rate and improve image quality.
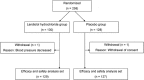
Methods: A total of 258 subjects suspected of having ischemic cardiac disease and requiring CCTA were randomized to either a landiolol hydrochloride 0.125 mg/kg group or placebo group to study the efficacy and safety of landiolol hydrochloride in a multicenter, double-blind, randomized parallel study. The primary endpoint was the diagnosable proportion (proportion of subjects whose coronary stenosis was diagnosable).
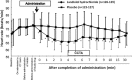
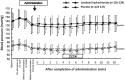
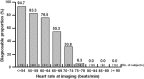
Results: The diagnosable proportions about the reconstruction images at mid-diastole were 68.2 and 38.2 % in the landiolol hydrochloride and placebo group, respectively, indicating significant superiority of landiolol hydrochloride over placebo (p < 0.0001). The diagnosable proportions about the optimal reconstruction images were 81.4 and 54.2 % in the landiolol hydrochloride and placebo group, respectively, indicating significant superiority of landiolol hydrochloride over placebo (p < 0.0001). The mean heart rate-lowering effect was first observed soon after administration of landiolol hydrochloride, was most marked at 3-5 min, and disappeared 30 min after completion of administration. The mean heart rate-lowering proportion at that time was -19.1 ± 8.1 % and -5.9 ± 9.7 % in the landiolol hydrochloride and placebo groups, respectively, showing a significantly higher proportion in the landiolol hydrochloride group.
Conclusions: Landiolol hydrochloride was confirmed to significantly and rapidly lower heart rate after intravenous injection, suggesting that it is a safe and useful agent for improving the image quality of CCTA.
Figures
Similar articles
-
A multicenter, open-label study of an intravenous short-acting β1-adrenergic receptor antagonist landiolol hydrochloride for coronary computed tomography angiography by 16-slice multi-detector computed tomography in Japanese patients with suspected ischemic cardiac disease.Drugs R D. 2014 Sep;14(3):185-94. doi: 10.1007/s40268-014-0056-6. Drugs R D. 2014. PMID: 25091378 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled, phase II dose-finding study of the short acting β1-blocker, landiolol hydrochloride, in patients with suspected ischemic cardiac disease.Int J Cardiovasc Imaging. 2013 Jun;29 Suppl 1(Suppl 1):7-20. doi: 10.1007/s10554-013-0253-3. Epub 2013 Jun 20. Int J Cardiovasc Imaging. 2013. PMID: 23784548 Free PMC article. Clinical Trial.
-
Dose-finding study of landiolol hydrochloride: a short-acting β1-blocker for controlling heart rate during coronary computed-tomography angiography in Japan.Adv Ther. 2013 Sep;30(9):803-18. doi: 10.1007/s12325-013-0053-0. Epub 2013 Sep 24. Adv Ther. 2013. PMID: 24062147 Free PMC article. Clinical Trial.
-
Landiolol: A Review in Tachyarrhythmias.Drugs. 2018 Mar;78(3):377-388. doi: 10.1007/s40265-018-0883-9. Drugs. 2018. PMID: 29470800 Review.
-
Evaluating the Therapeutic Efficacy and Safety of Landiolol Hydrochloride for Management of Arrhythmia in Critical Settings: Review of the Literature.Vasc Health Risk Manag. 2020 Apr 3;16:111-123. doi: 10.2147/VHRM.S210561. eCollection 2020. Vasc Health Risk Manag. 2020. PMID: 32308404 Free PMC article. Review.
Cited by
-
Bolus application of landiolol and esmolol: comparison of the pharmacokinetic and pharmacodynamic profiles in a healthy Caucasian group.Eur J Clin Pharmacol. 2017 Apr;73(4):417-428. doi: 10.1007/s00228-016-2176-0. Epub 2017 Jan 13. Eur J Clin Pharmacol. 2017. PMID: 28091703 Clinical Trial.
-
Safety of Landiolol Hydrochloride as a Premedication for Producing an Appropriate Heart Rate for Multidetector-Row Computed Tomography Coronary Angiography.J Clin Med Res. 2018 Jan;10(1):22-26. doi: 10.14740/jocmr3213w. Epub 2017 Dec 1. J Clin Med Res. 2018. PMID: 29238430 Free PMC article.
-
The Role of Landiolol in Coronary Artery Disease: Insights into Acute Coronary Syndromes, Stable Coronary Artery Disease and Computed Tomography Coronary Angiography.J Clin Med. 2025 Jul 23;14(15):5216. doi: 10.3390/jcm14155216. J Clin Med. 2025. PMID: 40806838 Free PMC article. Review.
-
A multicenter, open-label study of an intravenous short-acting β1-adrenergic receptor antagonist landiolol hydrochloride for coronary computed tomography angiography by 16-slice multi-detector computed tomography in Japanese patients with suspected ischemic cardiac disease.Drugs R D. 2014 Sep;14(3):185-94. doi: 10.1007/s40268-014-0056-6. Drugs R D. 2014. PMID: 25091378 Free PMC article. Clinical Trial.
-
Effect of Landiolol Hydrochloride on Hemodynamics in a Histamine-Induced Shock Model.Drugs R D. 2021 Sep;21(3):321-329. doi: 10.1007/s40268-021-00354-3. Epub 2021 Jun 14. Drugs R D. 2021. PMID: 34128200 Free PMC article.
References
-
- Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118:586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. - DOI - PubMed
-
- American College of Cardiography Foundation Task Force on Expert Consensus Documents. Mark DB, Berman DS, Budoff MJ, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;2010(121):2509–2543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

